Third reported patient with RAP1B-related syndromic thrombocytopenia and novel clinical findings
Dana Miller and Azhar Saeed are considered joint first author.
Matthew Bower and Anjali Aggarwal are considered joint last author.
Abstract
RAP1B is a RAS-superfamily small GTP-binding protein involved in numerous cell processes. Pathogenic gain-of-function variants in this gene have been associated with RAP1B-related syndromic thrombocytopenia, an ultrarare disorder characterized by hematologic abnormalities, neurodevelopmental delays, growth delay, and congenital birth defects including cardiovascular, genitourinary, neurologic, and skeletal systems. We report a 23-year-old male with a novel, de novo RAP1B gain-of-function variant identified on genome sequencing. This is the third reported case which expands the molecular and phenotypic spectrum of RAP1B-related syndromic thrombocytopenia.
1 INTRODUCTION
RAP1B belongs to a superfamily of RAS-like small GTP-binding proteins involved in cell signaling. A germline variant in RAP1B (OMIM* 179530) was first reported in a patient who presented with Kabuki-like dysmorphology, growth and developmental delays, seizures, strabismus, congenital heart defect, right tibial shortening, and brachyphalangy (Bogershausen et al., 2015). Complementation studies demonstrated loss-of-function of RAP1B and defect in the MEK/ERK pathway. Subsequent identification of two additional patients with germline de novo gain-of-function missense RAP1B variants led to distinction of a second phenotype (Niemann et al., 2020). Clinical features described in these two patients include hematologic abnormalities (thrombocytopenia and lymphopenia), neurodevelopmental delays, growth delay, facial dysmorphism and congenital birth defects. We report a third patient with RAP1B-related syndromic thrombocytopenia: a 23-year-old male with a de novo RAP1B likely pathogenic variant identified on genome sequencing. This report describes and expands upon the genetic and phenotypic spectrum of RAP1B-related disorder.
2 CASE REPORT
The proband is a 23-year-old male with mild intellectual disability, bicuspid aortic valve, dilation of aortic root and ascending aorta, hearing loss, and long-standing thrombocytopenia with lymphopenia who presented to genetics clinic for evaluation. He weighed 70 kg with a BMI of 24.1 kg/m2. Height was 171 cm (23rd centile, Z score = −0.74) which is short for his mid-parental height (184 cm, 80th centile) and head circumference of 55.5 cm (Z = +0.10). Physical exam was significant for facial dysmorphism including short palpebral fissures, high nasal bridge, narrow external auditory canals, small tragus, thin upper lip and narrow and high arched palate (Figure 1). The patient has brachydactyly and cutaneous syndactyly of all the fingers and toes 2–3. He has chest asymmetry with documented history of pectus carinatum that improved over time.
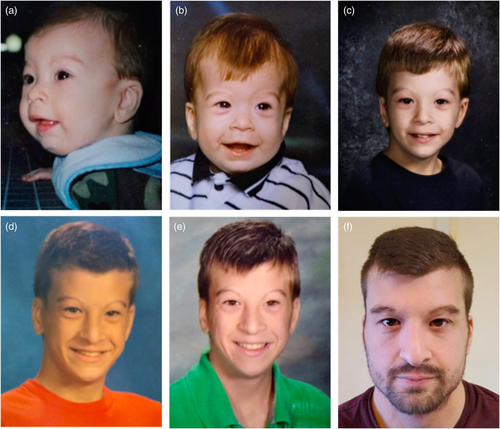
He was born at 38 weeks' gestation via vaginal delivery. Birth weight was 3.37 kg (37th centile, Z score = −0.34) and birth length was 54 cm (99th centile, Z score = +2.2). He has a history of postnatal microcephaly, although measurements are not available for review. He needed respiratory support in early neonatal period. Bronchoscopy examination identified tracheomalacia and aspiration. He failed newborn hearing screening and was found to have mild hearing loss with small, tortuous auditory canals and malformed auricles. Feeding difficulties and gastroesophageal reflux disease necessitated a Nissen fundoplication and gastrostomy tube (removed at 4 months of age). Renal ultrasound was normal. Hypotonia was noted in infancy, which resolved over time. He had frequent respiratory infections, seasonal allergies, and asthma in childhood. Sweat chloride testing was negative. He had an overbite and narrow palate, which required braces and expanders. His height through childhood was between 20th and 50th centile and weight between the 20th and 75th centile.
Developmentally, the patient experienced early motor delays requiring physical therapy. He first sat independently at 10 months and walked independently at 17 months of age. He began speaking at approximately 3 years of age and needed speech therapy. MRI of the brain performed at age 3 years showed hypoplasia of inferior cerebellar vermis. He continued to have difficulty with expressive language through age 5 years. He was diagnosed with learning disability and mild intellectual disability. He graduated from high school, received work skills training, and has worked at multiple department stores as a stocking employee.
At age 15 years, he developed a rash. Evaluation revealed thrombocytopenia, mild lymphopenia, and iron-deficiency anemia. Platelets at that time were 63 (ref 150–450 × 109/L) and lymphocyte count was 0.7 (ref 0.8–5.3 × 109/L). Bone marrow biopsy showed hypocellularity for age (40%–50%), erythroid predominant trilineage hematopoiesis, no dysplasia, and no increase in blasts; megakaryocytes had normal morphology and counts. Peripheral blood morphology confirmed slight polycythemia, slight leukopenia with absolute lymphocytopenia, and moderate thrombocytopenia. These were diagnosed to be idiopathic at that time. Asymptomatic thrombocytopenia and lymphopenia are persistent at 23 years of age. Anemia normalized with iron supplementation.
A screening echocardiogram was performed at 16 years of age due to a family history of aortic and brain aneurysms. He was found to have bicuspid aortic valve and aortic root enlargement measuring 35 mm at the sinuses of valsalva (Z score = +3 SD), 30 mm at the sinotubular junction, and 38 mm at the ascending aorta (Z score = +5 SD). He was started on Losartan at that time. At 23 years of age, chest magnetic resonance angiography (MRA) showed the aortic root was mildly dilated with maximal diameter of 4.2 cm (2.35 cm/m2) and ascending aorta mildly dilated with maximal diameter of 3.9 cm (2.18 cm/m2). EKGs have shown sinus bradycardia and nonspecific interventricular conduction delay. He exercises regularly and has been asymptomatic from a cardiac standpoint. MRA of the head was normal. The patient's vision is normal. Dermatologic history is significant for severe atopic dermatitis that has improved with biweekly Dupilumab.
Family history is significant for three siblings including one 18-year-old brother with ostial aneurysm of the right coronary artery. The other siblings have had negative echocardiograms. Father is well and had a negative echocardiogram. Maternal history is significant for high degree of myopia (−5.5 O.D, −5.25 O.S.), varicose veins, and negative echocardiogram. Maternal grandfather was reported to have learning difficulties, bicuspid aortic valve, 5 cm aortic aneurysm at age 60 years requiring repairs at age 60 and 71. Maternal great-grandfather died due to a brain aneurysm in his eighth decade of life. Maternal great-great aunts had dilated aortas, although details are unknown.
3 GENETIC TESTING
Prior genetic testing included negative chromosome analysis, negative array comparative genomic hybridization, and four next-generation sequencing panels which found no likely pathogenic or pathogenic variants.
Genome sequencing was performed on samples from the proband, mother, father, and brother as outlined in the Supporting Information. Variant prioritization identified a heterozygous, de novo missense variant in RAP1B (NM_001010942; c.176C>G (p.Ala59Gly) with greater than 20x coverage of the position in all four samples. This variant has not been described in the gnomAD control population database.
4 DISCUSSION
RAP1B is a member of the RAS family of small GTP binding proteins (GTPases), belonging specifically to the RAP subfamily. RAP1 occurs in two isoforms: RAP1A and RAP1B, encoded by RAP1A and RAP1B genes, respectively. Both proteins have 95% amino acid sequence identity, but with quantifiable differences in localization and function (Jaskiewicz et al., 2018). RAP1B is activated in many signal transduction pathways regulating numerous cellular processes including proliferation, adhesion, and migration. Various studies have highlighted its role in diverse contexts including neuronal differentiation, cytoskeleton assembly and formation, myogenesis, inflammatory response and homeostasis (Jaskiewicz et al., 2018).
The 35 members of the RAS subfamilies share the same functional domains as RAS and have highly similar primary sequence and thus changes in these highly conserved domains are expected to carry a similar biochemical and functional consequences across the RAS family members (Colicelli, 2004). All RAS-related proteins, including RAP1B, share a basic mechanism of operation enabling them to function as molecular switches in myriad signaling pathways, which relies on alternating between two conformations: an inactive GDP-bound state and an active GTP bound state.
The dynamics of the conformational states rely on structural changes confined to the switch I and switch II domains, which constitutes a major portion of the nucleotide binding pocket (Spoerner et al., 2001). The RAP1B variant identified in this case (p. Ala59Gly) is located at the start of the switch II domain, more specifically in the conserved DXXGQ motif crucial for GTP hydrolysis, Mg2+ coordination and nucleotide exchange (Lu et al., 2018). Noguchi et al. solved the crystal structure of wild type RAP1B in the active GppNHp (GTP analog) bound and inactive GDP bound state and highlighted that Alanine 59 is one of the critical residues influencing the switch I and switch II conformations in the physiological state (Noguchi et al., 2015). In addition, the glycine substitution at codon 59 of the highly related K- and H-RAS proteins has been shown by structural modeling to influence the conformations of the switch domains favoring an open switch I confirmation, and functionally results in impaired GAP-mediated GTP hydrolysis but with intact affinity to the RAS-binding domain of RAF proteins (Lu et al., 2018). This leads to increased downstream signaling through RAF/MEK/ERK cascade. Moreover, the glycine substitution at codon 59 has been characterized as a gain-of-function variant in KRAS and has been shown to be tumorigenic, operating through mitogen-activated protein kinase (MAPK) activation in laboratory studies (Kim et al., 2016). Based on these findings we concluded that the RAP1B variant found in this case (p. Ala59Gly) is a likely pathogenic gain-of-function variant.
Laboratory studies have shown that Rap1 proteins are involved in the regulation of MAPK pathway, exerting opposing effects on this pathway in cell-specific contexts, with activation effects operating through B-Raf and repressor effects operating through Raf-1 protein (Hu et al., 1997; Vossler et al., 1997). Laboratory studies on RAP1 germline loss-of-function variants (RAP1A p.Arg163Thr, RAP1B p.Lys151Glu) have shown that these variants cause dysregulation of the MAPK pathway and manifest as Kabuki-like syndrome (KL-S) with features including short stature, microcephaly, facial dysmorphism, developmental delay, and seizures. Although there are some overlap with the clinical features in our patient, thrombocytopenia was not part of the clinical features of KL-S (Bogershausen et al., 2015).
RAP1B activating germline variants have been described by Niemann et al. who first described two RAP1B-related syndromic thrombocytopenia patients (Niemann et al., 2020). Our patient's p.Ala59Gly variant also leads to gain-of-function as demonstrated by biochemical effects of this specific variant in the highly conserved switch II domain of all RAS-family proteins (Kim et al., 2016; Lu et al., 2018; Noguchi et al., 2015).
The Niemann et al. patients share common features with our patient including thrombocytopenia with leucopenia and lymphopenia, mild intellectual disability with or without learning disability, congenital brain malformations, and dysmorphic features (Niemann et al., 2020) (Table 1). Our patient's hypotonia, feeding issues in infancy, developmental delay, congenital heart defect, and short stature were consistent with the previously reported features in RAP1B-related syndromic thrombocytopenia. Our patient did not experience genitourinary malformations, puberty delay, or ocular anomalies, indicating these features are open to variable expressivity in this syndrome (Niemann et al., 2020). Our patient demonstrated multiple features that have not been reported in RAP1B-related syndromic thrombocytopenia patients including mild sensorineural hearing loss, speech delay, bite abnormality, and high arched palate. These features are consistent with other RASopathies, and activating RAS/ERK/MEK pathway dysregulation is characteristic of the RASopathies, supporting the inclusion of RAP1B-related syndromic thrombocytopenia as a RASopathy (Cao et al., 2017; Motta et al., 2020; Roberts et al., 2007; van Trier et al., 2015).
| Niemann et al. (2020) | Current case | Affected cases (n/total) | ||
|---|---|---|---|---|
| Patient 1 | Patient 2 | |||
| Genotype (RAP1B NM_001010942) | c.35G>T p.(Gly12Val), de novo | c.178G>C p.(Gly60Arg), de novo | c.176C>G p.(Ala59Gly), de novo | |
| Sex | Female | Male | Male | |
| Age at report (years) | 36 | 13 | 23 | |
| Dysmorphic features | Up slanting palpebral fissures, flat mid face, scarce eyebrows, low-set posteriorly rotated ears, preauricular tag | Hypertelorism, anteverted nostrils, long philtrum and thin upper lip, low-set posteriorly rotated ears | Short palpebral fissures, thin arched eyebrows, periorbital fullness, high nasal bridge, narrow auditory canals, small tragus, thin upper lip, narrow chin | 3/3 |
| Neurologic | ||||
| Hypotonia | + | − | + | 2/3 |
| Motor delay | + | − | + | 2/3 |
| Speech delay | − | − | + | 1/3 |
| Intellectual disability (degree) | + (mild) | ND | + (mild) | 2/3 |
| Learning disability | + | + | + | 3/3 |
| Postnatal microcephaly | + (no OFC) | + (OFC −2.5 SD) | History of microcephaly earlier in life, but OFC not available. No microcephaly currently | 2/3 |
| Abnormal brain imaging | + (aplasia of anterior septum pellucidum, partial cyclop ventricle, hypoplasia of hypothalamic structures and pituitary, abnormal sphenoid sinus with hyperintense structures) | + (nodular heterotopias of the right ventricle and hypoplasia of the cerebellar vermis) | + (Hypoplasia of inferior cerebellar vermis) | 3/3 |
| Ocular abnormality | − | + (hypermetropia, astigmatism) | − | 1/3 |
| Hearing loss | − | − | + (mild) | 1/3 |
| Dental abnormality | + (hypoplastic teeth) | − | + (overbite) | 2/3 |
| Endocrine | ||||
| Short stature | + (growth hormone deficiency and obesity [BMI 34.2 kg/m2]) | − | + (short for mid parental height) | 2/3 |
| Puberty delay | + (absent pubic/axillary hair, delayed pubarche/menarche) | ND | − | 1/3 |
| Cardiovascular | ||||
| Congenital heart defect | − | + (ventricular septal defect) | + (bicuspid aortic valve) | 2/3 |
| Aortopathy | − | − | + (aortic root and ascending aortic dilation) | 1/3 |
| Feeding difficulties in infancy | + | − | + (required G-tube till 4 months of life) |
2/3 |
| Genitourinary | ||||
| Renal hypoplasia/ agenesis | + (unilateral cystic renal hypoplasia) | + (unilateral renal agenesis) | − | 2/3 |
| Obstructive hydroureter | + | − | − | 1/3 |
| Musculoskeletal | ||||
| Brachydactyly | + | ND | + | 2/3 |
| Skeletal anomalies | + (congenital hip dysplasia) | ND | + (narrow/high arched palate, cutaneous syndactyly of all fingers and of toe, chest asymmetry, pectus carinatum) | 2/3 |
| Hematology | ||||
| Thrombocytopenia (age of diagnosis, count in 10e9/L) | + (14 months, 30–50) | + (congenital, <20) | + (15 years, 56–99) | 3/3 |
| Lymphopenia (age of diagnosis, count in 10e9/L) | + (ND, ND) | + (13 years, 1.3) | + (15 years, 0.6–1) | 3/3 |
| Leucopenia (age of diagnosis, count in 10e9/L) | + (ND, ND) | + (13 years, 3.8) | + (15 years, 3.6) | 3/3 |
| Anemia | + | − | + | 2/3 |
| Frequent infections | + (recurrent middle ear and nonhealing leg ulcers) | − | + (skin and respiratory infections in childhood) | 2/3 |
| Other | Splenomegaly, thin/dry skin, multiple nevi, hematomas | Bilateral inguinal hernias | Asthma, multiple allergies, atopic dermatitis, severe eczema, milia | |
- Abbreviations: +, present. −, absent; ND, not described; OFC, ocipitofrontal circumference.
The cardiac phenotype in our patient has not been described in the limited published cases, but aortic root aneurysm and bicuspid aortic valve have been reported in other RASopathies (Cornwall et al., 2014; Petersen et al., 2004). Our patient's aortic aneurysm and bicuspid aortic valve cannot be definitively ascribed to the RAP1B variant considering the de novo nature of the variant and the family history of similar concerns in his second-, third-, and fourth-degree relatives. Likewise, this feature cannot be ruled out as part of the phenotypic spectrum of RAP1B syndromic thrombocytopenia. Our patient's genomic data was reanalyzed to prioritize additional variants that might provide a unifying explanation for the personal and family history of bicuspid aortic valve and/or aneurysm. This additional analysis did not identify an additional diagnosis for the reported cardiac findings in our patient or family members.
RAP1 proteins have been described to play a role in the innate and adaptive immune response and regulation (Jaskiewicz et al., 2018). In addition, Rap1 has been found to regulate T-cell response by inducing anergy in the absence of costimulatory signal, through inhibition MAPK pathway by acting on Raf-1 protein. Rap1 deficient T cells have increased MAPK signaling in vitro, while an in vivo model of contact hypersensitivity demonstrated reduced TH-2 cytokine responses in Rap1 deficient mice (Dorn et al., 2012). While these findings might explain a causative link between the RAP1B variant described in our patient and the clinical finding of severe eczema, atopic dermatitis, and multiple allergies, they have not been reported to date in RAP1B-related syndromic thrombocytopenia published cases and may not be definitively ascribed to this disorder. Additional clinical observations and functional studies are needed to establish or refute these associations.
In summary, we report the third patient with a novel germline gain-of-function variant in the RAP1B gene associated with neurodevelopmental delays, congenital birth defects, lymphopenia and thrombocytopenia providing further evidence in support of gene-disease association. Mechanistic effects of variants in RAP1B-mediated RAS/ERK/MEK pathway seem to explain the two divergent phenotypes. Further studies are required to further understand the correlation between genotype and phenotype.
ACKNOWLEDGMENT
The authors of this paper would like to thank the patient and his family for their participation in this report.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Dana Miller: Writing – original draft; writing – review and editing. Azhar Saeed: Writing – original draft; writing – review and editing. Andrew C. Nelson: Writing – review and editing. Matthew Bower: Writing – review and editing. Anjali Aggarwal: Conceptualization; writing – review and editing.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




