Early-onset severe spinocerebellar ataxia 42 with neurodevelopmental deficits (SCA42ND): Case report, pharmacological trial, and literature review
Funding information: Agència de Gestió d'Ajuts Universitaris i de Recerca, Grant/Award Number: PERIS SLT008/18/00194; Instituto de Salud Carlos III, Grant/Award Number: National Grant PI17/00101; Ministerio de Ciencia e Innovación, Grant/Award Numbers: CEX2018-000792-M through the “María de Maeztu, RTI2018-094809-B-I00
Abstract
Early-onset severe spinocerebellar ataxia 42 with neurodevelopmental deficits (SCA42ND, MIM#604065) is an ultrarare autosomal dominant syndrome related to de novo CACNA1G gain-of-function pathogenic variants. All patients with SCA42ND show cerebellar atrophy and/or hypoplasia on neuroimaging and share common features such as dysmorphic features, global developmental delay, and axial hypotonia, all manifesting within the first year of life. To date, only 10 patients with SCA42ND have been reported with functionally confirmed gain-of-function variants, bearing either of two recurrent pathogenic variants. We describe a girl with congenital ataxia, without epilepsy, and a de novo p.Ala961Thr pathogenic variant in CACNA1G. We review the published subjects with the aim of better characterizing the dysmorphic features that may be crucial for clinical recognition of SCA42ND. Cerebellar atrophy, together with digital anomalies, particularly broad thumbs and/or halluces, should lead to clinical suspicion of this disease. We describe the first pharmacological attempt to treat a patient with SCA42ND using zonisamide, an antiepileptic drug with T-type channel blocker activity, in an off-label indication using an itemized study protocol. No efficacy was observed at the dose tested. However, without pharmacological treatment, she showed a positive evolution in neurodevelopment during the follow-up.
1 INTRODUCTION
Early-onset severe spinocerebellar ataxia 42 with neurodevelopmental deficits (SCA42ND, MIM#604065) is an ultrarare autosomal dominant syndrome first described in 2018 by Chemin et al. related to de novo CACNA1G gain-of-function pathogenic variants (Chemin et al., 2018). This syndrome is allelic to spinocerebellar ataxia 42 (SCA42, MIM#616795), an autosomal dominant adult-onset neurodegenerative disorder characterized by slowly progressive ataxia accompanied by other cerebellar signs (Morino et al., 2015). All patients with SCA42ND have cerebellar atrophy and/or hypoplasia on magnetic resonance imaging (MRI) and share common features such as global developmental delay and axial hypotonia, manifesting within the first year of life (Barresi et al., 2020; Chemin et al., 2018; Kunii et al., 2020). They also exhibit dysmorphic facial features and digital anomalies. Two recurrent CACNA1G pathogenic variants, p.Ala961Thr and p.Met1531Val, have been described in patients with SCA42ND. Moreover, Kunii et al. described a patient with an unreported heterozygous variant in CACNA1G (p.Ile1273Phe) whose pathogenicity remained undetermined after electrophysiological evaluation (Kunii et al., 2020). The description of the gain-of-function effect caused by the two recurrent pathogenic variants in the T-type CaV3.1channel raised the hypothesis that T-type channel blockers might have a therapeutic potential (Chemin et al., 2018), but such treatments have not been tested to date. However, some common antiepileptic drugs are known to act on T-type calcium channels (Powell et al., 2014). Here, we describe a girl with a de novo p.Ala961Thr pathogenic variant in CACNA1G and review the previously published subjects, in an attempt to better characterize the clinical picture to identify the key features for clinical recognition of SCA42ND. We also report the lack of efficacy of off-label administration of zonisamide (ZNS) in the context of an itemized study protocol.
2 CASE REPORT
The patient, a 25-month-old girl, was born after uneventful pregnancy and delivery to healthy, nonconsanguineous Spanish parents. She was born at 41 weeks and 4 days of gestation with a weight of 3,200 g (−0.3 SD), a length of 50 cm (0.0 SD), and an occipitofrontal circumference (OFC) of 35 cm (0.2 SD). The neonatal course was uneventful. At 4 months of age she was referred to early intervention services and pediatric ophthalmology due to axial hypotonia and strabismus. She achieved head control at 4 months of age. She was referred to our hospital at the age of 18 months, with normal height and weight but an acquired microcephaly (OFC 44 cm; −2.5 SD). She showed dysmorphic features including sparse, thin hair, high frontal hairline, broad forehead, deep-set eyes, upslanted palpebral fissures, retrognathia, and wide mouth (see all Human Phenotype Ontology [HPO] terms and iconographic details in Figure 1). Limb anomalies were also observed, including broad hallux, diffuse clubbing of toes, ulnar deviation of the second finger, prominent interdigital folds, fifth-finger clinodactyly, and thigh lipodystrophy (without inverted nipples); she presents a supernumerary nipple. Neurological examination revealed esotropia, axial hypotonia, normal strength and deep tendon reflexes, and no pyramidal signs. At that time, she had social smile, babbled, held hands together in the midline, and was unable to sit unsupported.
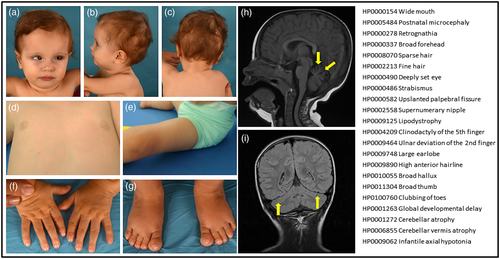
MRI revealed widening of the interfolia spaces predominantly in the superior vermis, denoting incipient cerebellar atrophy (Figure 1). General laboratory tests yielded normal results. Standard metabolic work-up for children with cerebellar atrophy (including isoelectric focusing of serum transferrin) showed normal results. A video-electroencephalogram and an array CGH were also normal. Trio-based whole exome sequencing (WES) identified a likely pathogenic de novo heterozygous missense pathogenic variant in the CACNA1G gene (NM_001256324, c.2881G>A, p.Ala961Thr), previously reported and classified as gain-of-function (Chemin et al., 2018).
Considering the unexplored potential benefit of T-type calcium channel blockers on SCA42ND (Chemin et al., 2018; Matar et al., 2009), ZNS was administered as an off-label treatment in the context of a 6-month study protocol accepted by the Local Pharmacy Commission. Still, although ZNS has been the last approved drug molecule with T-type channel activity for clinical use (Weiss and Zamponi, 2019), it also targets other channels and proteins to produce neuroprotection (Kopecky et al., 2014). Baseline evaluation included physical exam, cognitive assessment (Bayley Scales of Infant and Toddler Development III), language assessment (Communication and Symbolic Behavior Scales Developmental Profile [CSBS DP] Infant-Toddler Checklist), and motor assessment (Gross Motor Function Measure-88, GMFM-88). For the treatment of epilepsy, ZNS is usually initiated at 1–2 mg/kg/day and then increased until seizure control is reached (usual childhood maintenance dose is 4–8 mg/kg/day; Panayiotopoulos, 2010); given that our patient had no epilepsy, in the absence of adverse effects, our target dose was 2 mg/kg/day to be reached after slow titration at the 6th week. We selected that dose because low doses of 25 mg per day have been reported to be effective in the only previous report of an adult patient with SCA42 and severe tremor (Hara et al., 2019). At the 14th week, ZNS would be continued or discontinued, depending on the clinical response, and a final evaluation after withdrawal would also be informative. Follow-up evaluation included: vital signs, cognitive, social skills and language, and gross and fine motor at 5, 7, 14, and 24 weeks (unexpectedly the final evaluation had to be postponed until the 30th week due to COVID-19 isolation recommendations). Results are detailed in Table 1.
| Timeline | Baseline assessment | 5 weeks | 7 weeks | 14 weeks | 30 weeks |
|---|---|---|---|---|---|
| ZNS dosage | No treatment | 1.2 mg/kg/day | 2 mg/kg/day | 2 mg/kg/day | No ZNS |
| ZNS titration | After assessment started dosage at 0.2 mg/kg/day; slow titration, reached 1.2 mg/kg/day at the 4th week | Increased up to 2 mg/kg/day, reached at the 6th week |
Maintained |
ZNS was stopped abruptly after assessment |
|
| Age | 17 m 24 d | 19 m 10 d | 19 m 24 d | 21 m 15 d | 25 m 5 d |
| Cognitive assessment: Bayley-III cognitive subtest | |||||
| Direct score | 25 | 23 | 24 | 25 | 32 |
| Composite score | 55 | 55 | 55 | 55 | 55 |
| Percentile | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Equivalent age | 5 m 10 d | 5 m | 5 m | 5 m 10 d | 8 m |
| Language assessment: CSBS DP infant-toddler checklist (concern) | |||||
| Emotion and eye gaze | 6 | 7 | 7 | 8 | 8 |
| Communication | 6 | 7 | 8 | 7 | 7 |
| Gestures | 1 | 2 | 2 | 2 | 4 |
| Social composite | 13 (concern) | 16 (concern) | 17 (concern) | 17 (concern) | 19 (NOT concern) |
| Sounds | 4 | 5 | 7 | 7 | 7 |
| Words | 0 | 0 | 0 | 0 | 0 |
| Speech composite | 4 (concern) | 5 (concern) | 7 (concern) | 7 (concern) | 7 (concern) |
| Understanding | 4 | 4 | 4 | 3 | 4 |
| Object use | 3 | 3 | 3 | 3 | 3 |
| Symbolic composite | 7 (concern) | 7 (concern) | 7 (concern) | 6 (concern) | 7 (concern) |
| Total | 24 (concern) | 28 (concern) | 31 (concern) | 30 (concern) | 33 (concern) |
| Motor assessment: GMFM-88a | |||||
| Lying and rolling domain score | 25 | 31 | 31 | 31 | 51 |
| Lying and rolling domain percentile | 49.0 | 60.7 | 60.7 | 60.7 | 49.0 |
| Sitting domain score | 7 | 13 | 13 | 13 | 21 |
| Sitting domain percentile | 11.6 | 21.6 | 21.6 | 21.6 | 35.0 |
| Total score | 12.00% | 16.46% | 16.46% | 16.46% | 27.00% |
- Abbreviations: CSBS-DP, communication and symbolic behavior scales-developmental profile; GMFM-88, gross motor function measure-88; d, days; m, months; ZNS, zonisamide.
- a Gross Motor Function Measure (GMFM-88) consists of 88 items categorized into five domains: A. Lying and Rolling; B. Sitting; C. Crawling and Kneeling; D. Standing; E. Walking, Running, and Jumping. Results in the last three domains are not shown as they were always 0.
Written informed consent for genetic testing, publication, and off-label use of ZNS was obtained from the parents of the patient. Treatment with ZNS with slow titration was initiated at 17 months and 24 days of age at an initial dose of 0.2 mg/kg/day (once daily). Due to the absence of adverse effects, the ZNS dose was increased up to 2 mg/kg/day (twice daily) at the 6th week of the study protocol. At the 14th week, no significant improvement was observed in the main outcomes; physical therapists reported a mild improvement in axial tone, gaze control, cephalic control, and object manipulation with both hands, but GMFM-88 total score only increased 4.5% above the change expected due to spontaneous development (see Table 1). Consequently, treatment with ZNS was withdrawn at that point. At the 30th week, without ZNS treatment, still an overall improvement was observed in the main outcomes.
3 DISCUSSION
Here, we report the first Spanish patient with SCA42ND and review the previously published cases (Table S1) in an attempt to better document the frequent clinical characteristics of this disease and to identify the dysmorphic features and, particularly, digital anomalies that may be helpful clues for the diagnosis. This is the eleventh individual with SCA42ND reported in the literature (Barresi et al., 2020; Chemin et al., 2018; Kunii et al., 2020). For the first time, we describe the experience with off-label administration of ZNS in the context of an itemized study protocol.
All SCA42ND patients have been diagnosed in the context of trio-based WES analysis in cohorts of patients with cerebellar atrophy.
Overall, 3/11 patients are male and median age at initial signs/symptoms was 3 months of age (range 2 days to 6 months of age) (for more detail see Table S1). All patients show severe-to-profound developmental delay/intellectual disability with impairments in motor, language and social domains. Epilepsy has been reported in all the patients harboring the p.Met1531Val pathogenic variant (3/3), most of them with early-onset epileptic encephalopathy. In contrast, only one of the eight patients harboring the p.Ala961Thr pathogenic variant has developed epilepsy. The most common neurological findings are: cerebellar ataxia (10/11), distal hypertonia (8/11), axial hypotonia (8/11), strabismus (7/11), dysmetria (3/11), and nystagmus (2/11). All patients show MRI abnormalities, with the most common being global cerebellar atrophy or vermis atrophy.
In contrast with the relative non-specificity of these neurological manifestations, some characteristic dysmorphic features warrant special consideration, so as to avoid unnecessary delay in diagnosis. The most common dysmorphic facial features in the nine patients with available information are: microcephaly (3/9), high frontal hairline (9/9), sparse and/or thin hair (9/9), frontal bossing and/or broad forehead (7/9), deep-set eyes (5/9), upslanted palpebral fissures (5/9), short palpebral fissures (3/9), narrow nasal ridge and tip (3/9), prominent columella (2/9), hypoplastic alae (4/9), wide mouth (4/9), downturned corners of mouth (4/9), prognathism (3/9), retrognathia or micrognathia (2/9), and large earlobes (4/9). Regarding digital anomalies, 6/9 patients show fifth and/or second finger clinodactyly, 4/9 broad thumbs, and 4/9 broad halluces. We also observed thigh lypodystrophy, which, together with the cerebellar syndrome, raised the differential diagnosis of phosphomannomutase deficiency (PMM2-CDG) (Grünewald et al., 2002).
Overall, 8/11 patients with SCA42ND have the recurrent p.Ala961Thr pathogenic variant (including the present case) and 3/11 have the recurrent p.Met1531Val pathogenic variant, both located within the transmembrane segment S6 of CACNA1G (Barresi et al., 2020; Chemin et al., 2018) and leading to a gain-of-function effect. This raised the hypothesis that T-type channel blockers would have a therapeutic potential (Chemin et al., 2018). Interestingly, the blockade action of ZNS on T-type channels has been reported to rely on its capacity to enhance channel inactivation (Kito et al., 1996), therefore specifically counteracting one of the gain-of-function effects induced by pathogenic variant p.Ala961Thr and justifying its therapeutic use. Given that our patient did not have epilepsy, we considered off-label administration of ZNS for its known calcium channel blocker effect (Powell et al., 2014).
We report an itemized study protocol to objectively measure the benefit of a repurposed treatment administered to a single patient. Given the difficulties in launching clinical trials in ultrarare diseases, this approach might be a way to produce preliminary data, always considering risks and benefits for the patient. In order to objectively measure the potential benefit of a treatment in the developing child, it is important to use age-adjusted scales to take into consideration the expected change due to spontaneous development.
We did not observe a significant improvement in the main outcomes with the administration of ZNS at the dose tested. Its low blockade efficiency on human CaV3.1 channels (compared to that over T-type CaV3.2; Matar et al., 2009) may explain the insufficient efficacy of ZNS treatment in this study. Therefore, future studies might consider the administration of higher doses of ZNS or other drugs with known calcium channel blocker effect, such as ethosuximide and valproate (Powell et al., 2014), or new generation antiepileptic drugs with improved tolerability and safety. These antiepileptic drugs should also be taken into consideration in patients with CACNA1G-related epilepsy, with an eye toward an etiopathogenic effect. In this sense, for this type of ultra-rare disorders, the use of animal models is likely to be required to fully test and demonstrate the effect of such therapies.
To conclude, we suggest that cerebellar atrophy together with digital anomalies such as broad thumbs and/or halluces should lead to clinical suspicion of this disease, for which targeted therapies are likely to appear in the coming years.
ACKNOWLEDGMENTS
We thank the patient and her family for their kind collaboration. Mercedes Serrano is supported by the Generalitat de Catalunya (PERIS SLT008/18/00194) and National Grant PI17/00101 from the National R&D&I Plan, cofinanced by the Instituto de Salud Carlos III (Subdirectorate-General for Evaluation and Promotion of Health Research) and FEDER (European Regional Development Fund). José M. Fernández-Fernández is supported by the Spanish Ministry of Science and Innovation, the State Research Agency (Agencia Estatal de Investigación), and FEDER: Grants RTI2018-094809-B-I00, and CEX2018-000792-M through the “María de Maeztu” Programme for Units of Excellence in R&D to “Departament de Ciències Experimentals i de la Salut.”
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
The study was conceptualized and designed by Dídac Casas-Alba, Mercedes Serrano, Antonio F. Martínez-Monseny, Mercè Bolasell, and José M. Fernández-Fernández. Dídac Casas-Alba, Jordi Muchart, Laura López-Sala, Marta Pérez-Ordóñez, Rosanna Mari-Vico, and Mercedes Serrano acquired and analyzed the data. Mercedes Serrano, Antonio F. Martínez-Monseny, Mercè Bolasell, Dídac Casas-Alba, and José M. Fernández-Fernández drafted the manuscript and figures. All authors revised, corrected and approved the final report.
Open Research
DATA AVAILABILITY STATEMENT
Data is available if requested by editors.




