Non-syndromic anophthalmia/microphthalmia can be caused by a PORCN variant inherited in X-linked recessive manner
Abstract
Anophthalmia and microphthalmia (A/M) represent severe developmental ocular malformations, corresponding, respectively, to absent eyeball or reduced size of the eye. Both anophthalmia and microphthalmia may occur in isolation or as part of a syndrome. Genetic heterogeneity has been demonstrated, and many genes have been reported to be associated with A/M. The advances in high-throughput sequencing have proven highly effective in defining the molecular basis of A/M. Nevertheless, there are still many patients with unsolved genetic background of the disease, who pose a significant challenge in the molecular diagnostics of A/M. Here we describe a family, with three males affected with the non-syndromic A/M. Whole exome-sequencing performed in Patient 1, revealed the presence of a novel probably pathogenic variant c.734A>G, (p.[Tyr245Cys]) in the PORCN gene. Pedigree analysis and segregation of the identified variant in the family confirmed the X-linked recessive pattern of inheritance. This is the first report of X-linked recessive non-syndromic A/M. Until now, pathogenic variants in the PORCN gene have been identified in the patients with Goltz syndrome, but they were inherited in X-linked dominant mode. The ocular phenotype is the only finding observed in the patients, which allows to exclude the diagnosis of Goltz syndrome.
1 INTRODUCTION
Anophthalmia (MIM 206900) and microphthalmia (MIM 309700) are the most severe malformations of the eye. Anophthalmia corresponds to the total absence of the ocular tissue (true anophthalmia) or absence of the visible ocular structures with remnants that can be histologically detectable (clinical anophthalmia; Plaisancié et al., 2019). Microphthalmia refers to a decreased size of the eye, clinically confirmed if a total axial length is more than two standard deviations below the mean for age (<21 mm in adults and <14 mm in newborns; Verma & FitzPatrick, 2007). The prevalence of A/M is estimated as being between 1 and 3 per 10,000 live births (Clementi et al., 1996; Forrester & Merz, 2006; Källén, Robert, & Harris, 1996; Roos, Jensen, Grønskov, Holst, & Tümer, 2016). Both anophthalmia and microphthalmia may occur as an isolated finding without systemic features or as a part of a syndrome and can be unilateral or bilateral. A/M has a complex etiology that includes genetic and environmental causes. However, genetic defects are thought to account for the majority of A/M cases. More than 30 genes are implicated in the pathogenesis of the non-syndromic A/M. The main causative genes are: SOX2 (MIM 184429), OTX2 (MIM 600037), RAX (MIM 601881), FOXE3 (MIM 6011094), and PAX6 (MIM 607108) (Bardakjian & Schneider, 2011; Verma & FitzPatrick, 2007). Currently, mutations in known genes are identified in <40% of A/M cases, therefore additional causative genes need to be discovered (Bardakjian & Schneider, 2011; Slavotinek, 2019).
We present, the first family (three males affected) with the non-syndromic X-linked recessive A/M caused by the variant in the PORCN gene. So far, pathogenic changes of the PORCN gene have been shown to be associated with X-linked dominant Goltz syndrome (GS) only, known also as focal dermal hypoplasia (MIM 305600; Bostwick, Van den Veyver, & Sutton, 1993; Goltz, 1992).
2 PATIENTS AND METHODS
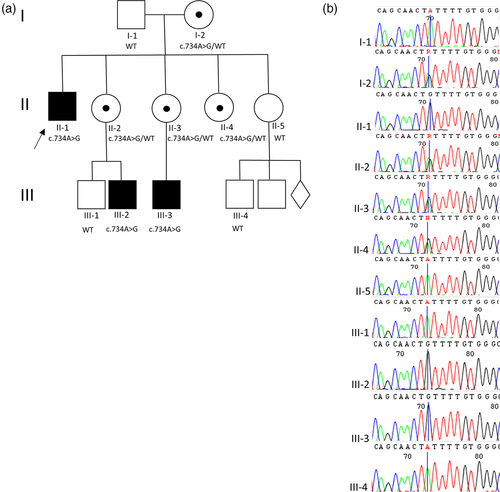
Three male patients from one family of Polish origin affected with anophthalmia/microphthalmia were recruited to this study (Figure 1). The patients' parents do not show any symptoms of A/M or any other ocular and congenital abnormalities. Written informed consent was obtained from the family prior to molecular testing. All procedures were performed under the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Genomic DNA was extracted from peripheral blood leukocytes and from buccal cells. Blood samples were collected from the Patient 1 and nine healthy family members. Genomic DNA was extracted from the peripheral blood lymphocytes using the MagCore HF16 Automated Nucleic Acid Extractor and Genomic DNA Large Volume Whole Blood Kit (RBC Bioscience Corp.). DNA from buccal cells was isolated from Patients 2 and 3 according to a kit protocol (Nucleo-Spin Tissue, Machery-Nagel).
A DNA sample from Patient 1 was subjected to the exome capture and high-throughput sequencing. Target enrichment was performed using the Agilent SureSelectXT Human All Exon V6 kit and sequencing of 100 bp paired-end reads was carried out on Illumina HiSeq 1500. A variant-discovery pipeline was built based on the GATK Best Practices. The human reference genome (hg 19) was used. Filtering and interpretation of NGS data was done using Exomiser running the hiPHIVE algorithm. Variants were visualized with the use of Integrative Genomics Viewer (IGV). The following Human Phenotype Ontology (HPO) terms were used as the input: anophthalmia, microphthalmia and abnormal eye morphology. To confirm the presence of the identified variant, the standard polymerase chain reaction (PCR) and Sanger sequencing was performed. Sequencing results were aligned with the reference sequence of the PORCN gene (NM_022825.3) and were analyzed using CodonCode Aligner software.
3 RESULTS
3.1 Clinical report
3.1.1 Patient 1
A 38-year-old patient was a proband initially diagnosed as isolated sporadic anophthalmia of unknown etiology. He was born from normal pregnancy and uneventful labor in the 42nd week of gestation with birth weight of 3,800 g (90th percentile) and Apgar score 10. Directly after birth congenital absence of the eyeballs was suspected. Precise ophthalmological and ultrasound examination allowed to find a soft and translucent anophthalmic cyst (approximately 12 mm in diameter) everted out of the right orbit together with conjunctiva of the lower eyelid. At the left side a small and translucent remnant of an underdeveloped eye (approximately 5 mm in diameter) was found. No elements of the anterior segment of the eyes could be found. The anophthalmic cyst was surgically excised and the patient was referred for the eyeball prosthesis adjustment. The psychomotor development was normal and until now there are no other chronic health problems.
3.1.2 Patient 2
Patient 2 is a 5-year old male, born in the 40th week of gestation from normal pregnancy and uneventful labor. He is the second child of unrelated parents. His birth weight was 3,250 g (50th percentile). Shortly after birth during ophthalmologic examination rudimentary right eyeball has been found and in the left eye no elements of anterior segment of the eye could be distinguished. Flash visual evoked potentials (FVEP) test performed at the age of 1 month revealed low amplitude recordings with rudimentary P1, P2, and P3 waves in the right eye. Magnetic resonance imaging (MRI) at the age of 3 weeks revealed a cyst located in the inferionasal area of the left orbit moving the eyeball subtemporally and modeling inferior rectus and medial rectus muscles. Another small cyst was located behind the eyeball. Two small cysts located behind the rudimentary eyeball were identified in the right orbit. Cysts are communicating with the eyeball in the proximity of the optic nerve. Both eyeballs are small and show an abnormal shape. Right optic nerve seems normal but the left optic nerve is modeled by the cyst in its intraorbital segment. In the white matter of occipital lobes there were massive hypoxic–ischemic changes and on the cerebellar hemispheres level the subdural hemorrhages were observed. The child shows psychomotor developmental delay, that is assessed by pediatric neurologist as caused by the hypoxic–ischemic changes in the CNS.
The patient was referred for the eyeball prosthesis (expander) adjustment and did not attend the next fixed control visits.
3.1.3 Patient 3
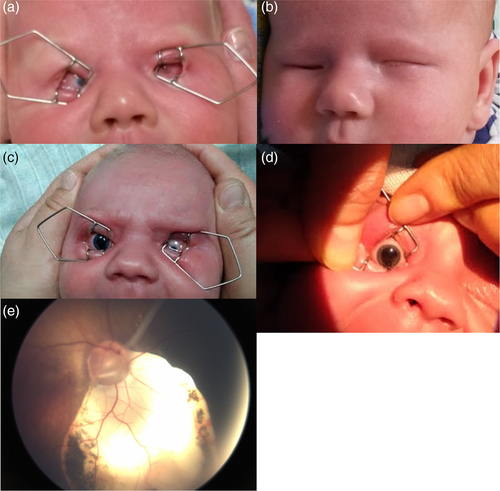
This 4-month-old male patient was born in the 41st week of gestation, his birth weight was 3,550 g (75th percentile). The ophthalmological examination allowed to find a microphthalmic right eye with inferionasal uveal coloboma comprising iris, ciliary body, choroid and the optic nerve. The left eye was also microphthalmic with evident microcornea (Figure 2). Due to lack of fixation, there was no possibility to measure the refractive error of the eyes. Reaction to the light was preserved, although disturbed. Flash visual evoked potentials (FVEP) test revealed low amplitude and prolonged latency P2 wave in the right eye. The ultrasound examination of the eyes and orbits showed that both eyeballs are small with large inferionasal coloboma with retina attached in the right eye and with total retinal detachment (closed funnel) but no coloboma in the left eye. Behind the right eyeball, in the inferionasal area of the orbit there was a hypoechogenic area (cyst). Transfontanellar and abdominal ultrasound examination gave normal results. The final diagnosis was colobomatous microphthalmia of the right eye and microphthalmia with total retinal detachment in the left eye. The child was referred for visual rehabilitation, head and orbit MRI and next ophthalmological control visit at the age of 6 months.
3.2 Molecular report
A whole-exome sequencing was performed for Patient 1 in order to determine the molecular cause of A/M. The missense variant c.734A>G, (p.[Tyr245Cys]) in the PORCN gene was revealed in the hemizygous state in the three male patients and in the heterozygous state in four females by Sanger sequencing (Figure 1). Thus, the identified variant cosegregates within the family. The novel missense change was not listed neither in the Human Gene Mutation Database (HGMD), NHLBI Exome Variant Server (EVS) nor in the Exome Aggregation Consortium (EXAC) browsers, and gnomAD database. Furthermore, it was predicted to be probably damaging in the in silico analyses performed by us with the use of Mutation Taster 2, PolyPhen2, and SIFT software. According to (ACMG) guidelines the variant was predicted to be likely pathogenic (Richards et al., 2015).
4 DISCUSSION
Here, we present a family of Polish descent, with three males affected with a non-syndromic A/M. The molecular analysis evidenced a novel missense variant c.734A>G (p.[Tyr245Cys]) in the PORCN gene. Similar substitution in the PORCN gene has been reported by Leoyklang et al. in 2008. The authors identified a p.Arg228Cys variant in a female patient with Goltz syndrome. This variant was inherited from her unaffected mother. Moreover, a p.Ser247Glufs*315 variant has been found on the same allele in the affected individual indicating that this change has arisen de novo in the patient. The p.Arg228Cys substitution is predicted to be possibly damaging by Polyphen in silico analysis. These findings suggest that the p.Ser247Glufs*315 variant is likely associated with the patient's phenotype (Leoyklang, Suphapeetiporn, Wananukul, & Shotelersuk, 2008). The variant p.Tyr245Cys identified in our study is situated in the membrane bound O-acyl transferase, (MBOAT) protein domain. The position is evolutionary conserved (PhyloP score of 4.7 and GERP ++ score of 5.5) and there is a sizeable physiochemical difference between Tyr and Cys (Grantham Score is 194). Based on the bioinformatic analyses, as well as the type and location of the identified variant, we can assume its potential pathogenicity. PORCN gene (MIM 300651) located on the X chromosome (Xp11.23), belongs to the evolutionarily conserved porcupine (Porc) gene family. Porcupine endoplasmic reticulum proteins are involved in the processing of Wnt proteins, critical for interactions between ectoderm and mesoderm during embryogenesis. Until now, loss-of-function mutations in the PORCN gene have been implicated in X-linked dominant Goltz syndrome only (Durack et al., 2017; Grzeschik et al., 2007; Happle, 2016; Wang et al., 2007). Approximately 85–90% of patients are females with heterozygous or mosaic mutations. Males account for 10% of affected individuals and they always have mosaic mutations. Hemizygous non-mosaic mutations are thought to be lethal in males (Deidrick, Early, Constance, Stein, & Fete, 2016). In contrast to X-linked dominant inheritance described in Goltz syndrome, we present a novel variant in the PORCN gene found in all affected hemizygous males as well as in their unaffected, heterozygous carrier mothers, pointing to the X-linked recessive mode of inheritance (Figure 1). To our knowledge, this is the first description of the X-linked recessive inheritance of a PORCN variant that is not lethal for hemizygous males.
Goltz syndrome (known also as focal dermal hypoplasia, FDH), constitutes a rare multisystem genetic disorder with variable meso-ectodermal abnormalities, resulting in cutaneous, dental, craniofacial, skeletal, and ocular anomalies. Clinical findings concerning the eye include choroid or retinal coloboma, cataract and anophthalmia/microphthalmia occurring less frequently (Bostwick, Fang, Patel, & Sutton, 2016). Previous studies have noted that ophthalmologic symptoms are present in 40% of FDH patients (Goltz, Henderson, Hitch, & Ott, 1970; Thomas, Yoshizumi, Beyer, Craft, & Albert, 1977). Gisseman et al. in the study of 18 patients, showed a higher incidence of ophthalmologic anomalies in 77% of FDH patients (Gisseman & Herce, 2016). However, these data clearly indicate that the ophthalmologic manifestations are not present in all the FDH patients. In the index family, anopthalmia/microphthalmia phenotype is present in all affected family members and the symptoms are limited to the eyes only, which allows to exclude the diagnosis of Goltz syndrome. Purely ocular phenotype presented in patients can be partly explained by the observations on animal model. Bankhead et al. presented a mouse model with a deletion of Porcn gene, that shows high penetrance of ocular abnormalities similar to those found in FDH patients. They showed that Porcn is required in extraocular and neuroectodermal tissues for Wnt-dependent processes regulation, during morphogenesis of the posterior and anterior segments of the eye (Bankhead et al., 2015). Non-syndromic A/M has been associated with autosomal recessive and autosomal dominant patterns of inheritance so far. X-linked recessive inheritance in A/M spectrum disorders can be observed, but it applies to a syndromic form of the disease only, known as Lenz syndrome. Lenz microphthalmia syndrome (LMS), in addition to A/M spectrum, usually includes mild to severe developmental delay, skeletal abnormalities, genitourinary malformations, and anomalies of the digits, ears, and teeth (Bardakjian, Schneider, Ng, Johnston, & Biesecker, 2009; Traboulsi et al., 1988). Two genes are known to be involved in LMS pathogenesis: BCOR (BCL-6 corepressor, MIM 300485) and NAA10 (N-acetyltransferase, MIM 300013) (Esmailpour et al., 2014; Ng et al., 2004). The clinical symptoms observed in our patients, as well as the causative variant identified in the PORCN gene and normal results of the BCOR and NAA10 analysis, allow to exclude Lenz syndrome in the presented family. In consequence, we can assume that this is the first evidence of an isolated X-linked recessive A/M caused by a variant in the PORCN gene.
In conclusion, our findings show for the first time that a hemizygous variant in the PORCN gene is not lethal for the affected males and shows familial occurrence. It can be inherited in the X-linked recessive manner and can be responsible for a non-syndromic A/M spectrum—from anophthalmia and anophthalmic cyst to various forms of microphthalmia. Our findings stay in agreement with the observations concerning intrafamilial phenotypic variability among the patients with A/M spectrum, that can be associated with other modifying genetic or non-genetic factors.
ACKNOWLEDGMENT
We thank the family for their participation in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




