Clinical characteristics and rate of dilatation in Turner syndrome patients treated for aortic dilatation
Abstract
Turner syndrome is associated with an increased risk of aortic aneurysms and dissection. Recent 2017 clinical care guidelines recommend medical therapy to treat aortic dilatation, although whether this slows dilatation is unknown. We aimed to describe a pre-guideline cohort of Turner syndrome patients with aortic dilatation, the rate of dilatation following diagnosis, and post therapy dilatation rates. We conducted a retrospective review of Turner syndrome patients with a dilated aortic root or ascending aorta by current definitions. In total, 40 patients were included with 22 treated patients. Most patients had 45,X karyotype, were white, non-Hispanic, and received both growth hormone and estrogen. Except for hypertension, there were no differences in risk factors among treated and untreated groups. Bicuspid aortic valve was very common. Treatment group patients had significantly more dilated ascending aortas by absolute measurements and aortic size index. In an adjusted model, there was minimal change in aortic measures over time and this was not associated with medication use. In conclusion, in this cohort, Turner syndrome patients with aortic dilatation were more likely to be treated if they had hypertension and if they met multiple dilatation criteria. Further study is needed to establish medical therapy efficacy on dilatation progression.
1 INTRODUCTION
Turner syndrome is associated with a significantly increased risk of developing aortic aneurysms and dissection. Aortic dilatation has been reported in up to 4–42% of patients with Turner syndrome, and although dissection is rare (2–8% of all patients with Turner syndrome) (Ho et al., 2004), it is most often fatal (Lin, Lippe, & Rosenfeld, 1998). Thoracic aortic dissection occurs in Turner syndrome patients at a rate of ~36 per 100,000 person-years compared with 6 per 100,000 person-years in the general population (Gravholt et al., 2006). Dissection occurs most commonly in women with Turner syndrome at a median age of 35 years old with ranges from 4 to 64 years old, compared to a median age of 77 years old for females with ranges from 50 to 80 years old in the general population (Carlson & Silberbach, 2007; Gravholt et al., 2006). Certain risk factors such as bicuspid aortic valve, coarctation of the aorta, elongation of the transverse aorta, or hypertension are thought to increase the risk of dilatation and dissection (Carlson, Airhart, Lopez, & Silberbach, 2012; Duijnhouwer et al., 2018; Lin et al., 1998; Lopez et al., 2008; Matura, Ho, Rosing, & Bondy, 2007; Mortensen, Erlandsen, Andersen, & Gravholt, 2013; Olivieri et al., 2013; Quezada, Lapidus, Shaughnessy, Chen, & Silberbach, 2015; Turtle, Sule, Webb, & Bath, 2014). However, dissection has been documented in Turner syndrome patients without known risk factors (Lin et al., 1998), and dissections in children and adolescents with Turner syndrome have also been reported (Turtle et al., 2014).
Aortic dilatation typically precedes aortic dissection, and thus, recently-released Turner syndrome aortopathy consensus guidelines recommend monitoring aortic measurements, which are normalized to patient size due to the small stature of women with Turner syndrome (Gravholt et al., 2017; Matura et al., 2007; Silberbach et al., 2018). The aortic size index normalizes to body surface area and is used for patients >15 years of age. Those with an aortic size index ≥2.0 cm/m2 are designated as being higher risk for dissection, and therefore require close monitoring (Turtle et al., 2014). An aortic size index of ≥2.5 cm/m2 or a rapid increase over a 1-year period in a woman with Turner syndrome suggests the highest risk for dissection, so both medical therapy optimization and surgical referral are recommended (Carlson et al., 2012; Gravholt et al., 2017; Hjerrild et al., 2010). Recent evidence demonstrated that aortic size index is strongly age-dependent under ~15 years, thus Turner-specific Z-scores (body surface area-adjusted Z-scores for the aortic valve, aortic root, and aortic arch developed specifically for Turner syndrome patients) have been proposed to monitor aortic dilatation in children with Turner syndrome (Quezada et al., 2015). The rate of aortic dilatation in all Turner syndrome patients have been well-described (Alami Laroussi, Dahdah, Dallaire, Thérien, & Fournier, 2016; Duijnhouwer et al., 2018; Mortensen et al., 2011, 2013), but in Turner syndrome patients who specifically have been diagnosed with aortic dilatation, the risk factors and rate of dilatation are not well characterized.
Medical management for aortic dilatation is recommended under the current guidelines (Gravholt et al., 2017; Silberbach et al., 2018), but evidence for optimal regimens is lacking. Most treatment regimens are drawn from research in other genetically-mediated aortopathies (i.e., Marfan syndrome), where the use of beta blockers, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors have been studied or proposed. The ideal treatment regimen for other syndromic aortic aneurysm conditions is still the subject of ongoing research (Hofmann Bowman, Eagle, & Milewicz, 2019). Furthermore, whether medications are effective in slowing the rate of aortic dilatation in Turner syndrome is unknown.
In this cohort of Turner syndrome patients with diagnosed aortic dilatation, we describe their clinical and treatment characteristics, the rate of dilatation following aortic dilatation diagnosis, and the rate of dilatation among those on medication.
2 MATERIALS AND METHODS
2.1 Study population
This was a retrospective cohort study consisting of a review of existing medical records from 2004 through 2018 at a single pediatric academic center with a large referral population for an endocrinology-specific Turner syndrome clinic. Turner syndrome patients with cardiovascular disease are followed at the referral center by a pediatric cardiology clinic specializing in cardiovascular genetics or by pediatric cardiologists, either at the referral center or locally. Patients diagnosed with both Turner syndrome and aortic dilatation were identified by query of the electronic medical record (EMR, Epic Systems Corporation, Verona, WI). This list was cross-referenced with patients independently classified in an internal Turner syndrome database to have aortic dilatation or risk factors for dilatation, including coarctation of the aorta, bicuspid aortic valve, hypoplastic aortic arch, aortic valve disease, or hypertension. Patients with both Turner syndrome and aortic dilatation identified before April 1, 2017 were eligible for inclusion. In accordance with current Turner syndrome recommendations (Silberbach et al., 2018), dilatation was defined as (a) Turner-specific Z-score ≥ 3 if ≤15 years old, (b) aortic size index ≥2.0 cm/m2 if >15 years old, or (c) absolute aortic root diameter > 3.3 cm or ascending aortic diameter > 3.1 cm by either transthoracic echocardiogram or cardiac magnetic resonance imaging (MRI). In order to standardize the cohort, the patients who had been diagnosed with aortic dilatation based on absolute measurement or non-Turner-specific Z-scores (Colan, 2009), but would not have met criteria by the current aforementioned Turner syndrome-specific recommendations for defining aortic dilatation were excluded. Patients without imaging sufficient to confirm or follow aortic dilatation were also excluded. The study was approved by the Cincinnati Children's Hospital Medical Center Institutional Review Board.
The data collected from the EMR included demographics, karyotype, treatment with growth hormone and/or estrogen (with treatment start and end dates, if available), hypertension at the time of diagnosis and if treated, medication type, dose, and compliance; presence of and type of bicuspid aortic valve, presence of and severity of aortic valve stenosis, presence of and severity of aortic valve regurgitation, history of coarctation of the aorta and type of repair, presence of elongation of the transverse aorta, and other co-existing cardiac anomalies. At the time of diagnosis by transthoracic echocardiogram or MRI, as well as at every subsequent imaging visit, data extracted from the EMR included weight, height, blood pressure, and treatment for aortic dilatation (including specific type of medication, dose, and compliance). All imaging visits, regardless of aortic dilatation treatment status, between the time of diagnosis and the end of the study period were included, or until the patient underwent surgical intervention on aortic dilatation.
2.2 Transthoracic echocardiogram measurements
From each transthoracic echocardiogram, the aortic valve annulus, aortic root, sinotubular junction, and ascending aorta diameters were measured. Aortic measurements were obtained with two-dimensional echocardiography in the parasternal long axis in systole, measured from inner edge to inner edge perpendicular to the long axis of the vessel at maximal expansion in systole, according to consensus recommendations (Lopez et al., 2010).
2.3 Cardiac MRI measurements
From each cardiac MRI, maximal systolic measurements within the sinuses of Valsalva of the aortic root were measured from the acquired aortic root short axis steady-state free precession (SSFP) cine stack images. The three cusp-to-commissure and the three cusp-to-cusp diameters were measured at the aortic root level according to the method indicated by Rodríguez-Palomares et al., using the inner edge—inner edge convention (Rodríguez-Palomares et al., 2016). Each bicuspid aortic valve was classified according to the Sievers system (Sievers & Schmidtke, 2007). In the setting of the bisinuate aortic root seen in the bicuspid aortic valve without raphe (Sievers classification Type 0), the maximal longitudinal and minimal transverse diameters were measured using this method at the aortic root level (Vis et al., 2019). For the ascending aorta cardiac MRI measurements, the short axis diameters were obtained at the level of the right pulmonary artery using the double-oblique method with multiplanar reconstruction of the 3D contrast-enhanced SSFP or mDixon magnetic resonance angiography sequence which was cardiac gated in systole and navigated to the diaphragm (Mortensen et al., 2011). At maximal size, anteroposterior and laterolateral diameters were measured using the inner edge—inner edge convention. To improve reliability, aortic dimensions were measured by a single reader (C.P.) and 10% were re-measured by a second reader (J.T.). The aortic dimensions were each averaged, with the mean values used in statistical analysis as absolute measurements. The averaged cusp-to-cusp MRI measurement was preferentially used over the cusp-to-commissure measurements as it has been shown to correlate better with transthoracic echocardiogram aortic root measurements (Vis et al., 2019), after both were evaluated and found to be internally consistent. Body surface area was approximated by the Haycock formula (Haycock, Schwartz, & Wisotsky, 1978), and was then used to generate aortic size indexes (absolute measurement divided by body surface area) and Turner-specific Z-scores, the latter utilizing normative equations per Quezada et al. (Quezada et al., 2015).
From the medication data extracted at imaging visits, patients were divided into two groups: therapy or no therapy. If treated with medications for aortic dilatation for at least 6 months at any point during the study period, patients were included in the therapy group. The patients who were prescribed medications for aortic dilatation but had reported noncompliance with less than half of their prescribed doses per week or who were on medications for less than 6 months were categorized as not receiving therapy.
2.4 Statistical analysis
The analyses were performed using JMP statistical software package (SAS Institute, Cary, NC). Data were evaluated for distributional qualities. The inter-observer measurement variability was low, with strong correlation (R2 > 0.95 for echo and R2 > 0.85 for MRI) between readers on the independently reviewed studies.
The baseline characteristics of the cohort were described. Because some continuous variables exhibited deviations from normality, medians and inter-quartile ranges are reported. Categorical data are reported as frequencies. To identify differences between groups, we used Wilcoxon rank sums (continuous non-normally distributed variables) and Fisher exact test and χ2 goodness of fit tests (categorical variables).
In this study, we sought to determine if medication use was associated with rate of change in aortic measures. It is well recognized that aortic size is related to age and body surface area, and both the Turner-specific Z-score and the aortic size index were developed to account for these factors when considering aortic size measures. However, since these measures are age-specific, neither adjusted index was sufficient for our broad age range. Further, these indexed measurements have different units of measure. Therefore, we did not use them for statistical analysis as continuous variables. Instead, we created adjusted aortic root and adjusted ascending aortic measures. To develop these adjusted measures, we restricted the data to individuals not on medication. We ran mixed models, with subject as a random effect and adjusted for age, age2, body surface area, imaging modality, and presence of hypertension, bicuspid aortic valve, and coarctation of the aorta. Although only age and age2 had statistically significant effects as single variables within the predictive models, all variables were included in the predictive model due to their clinical importance. The predictive equation from each model (ascending aorta and root) was then applied to all data and residuals were generated. These residuals are our adjusted ascending aorta and root measures. As individuals had variable follow up, both in frequency and duration, change since baseline adjusted aortic measures were calculated.
The mean absolute ascending aorta measurements, as well as the difference in these measurements over time, were also calculated using combined echocardiography and MRI data to assess for unadjusted rate of dilatation for the cohort and between treatment groups using linear mixed models with individual as a random effect.
To test whether medication was associated with a differential change in aortic measures over time, we again used linear mixed models with individual as a random effect. The primary outcome variables were adjusted changes in the aortic root and ascending aorta absolute measurements over time. Predictors of our outcome variable were time since baseline, medication use, and an interaction between time since baseline and medication use. As medical treatment is a time-varying covariate, the interaction specifically tested whether the rate of change since baseline differed after medication initiation. Least square means for medication use were estimated to account for other covariates in the model. A sub-analysis was performed to assess whether these indices differed by patient age.
3 RESULTS
The medical histories of the 55 patients identified as having a diagnosis of both Turner syndrome and aortic dilatation were reviewed (Figure 1). After excluding those who had insufficient imaging for analysis (only one study available for review; five patients) and those who did not meet the diagnostic criteria of dilated aorta by current Turner syndrome definitions at any point during the study period (those who had been diagnosed with aortic dilatation based on lower absolute measurement or non-Turner-specific Z-scores; 10 patients), there were 40 patients who comprised the study population, with imaging studies from 2004 through 2018.
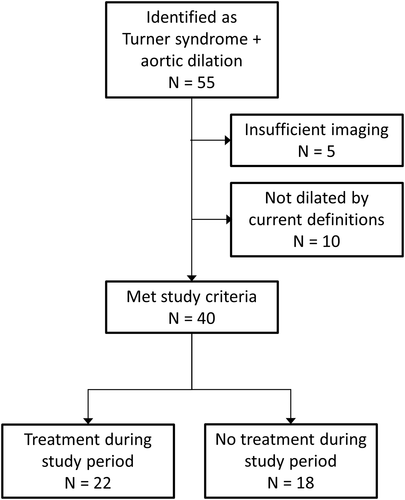
Following the diagnosis of aortic dilatation, there was a median of three transthoracic echocardiograms per subject (range 0–9) and a median of two MRI studies per subject (range 0–4) with a total median of five imaging studies per subject (range 2–12). There were 22 patients who were treated with medications for aortic dilatation at any point during the study period and 18 patients who were considered untreated, including one patient with reported noncompliance. Two patients in the treatment group underwent surgical repair of severe ascending aortic dilatation, at which point medications were discontinued and their subsequent aortic dimension data were not included. No patients experienced aortic dissection during the study period.
The study population consisted predominately of white, non-Hispanic females in adolescence at diagnosis (Table 1), similar to the regional population. The median age at diagnosis was 15.3 years (range 9.6 to 20.6 years), with a median age at follow-up of 21.2 years (range 14.8 to 29.0 years). There were no significant demographic differences between the patients treated for aortic dilatation and those untreated. The most common karyotype was 45,X, with other karyotypes including four patients with mosaic 45,X/46,XX, three patients with 46,XiX(q10), one patient each with mosaic 45,X/46,XY and 46,Xdel(X)(p22.1), and one karyotype was unknown. As seen in other cohorts with relatively young Turner syndrome populations (Uçar et al., 2018), treatment with growth hormone was common (78% of the patients). A similar percentage of patients (73%) were treated with estrogen.
| All | Treated | Untreated | p-value | |
|---|---|---|---|---|
| N | 40 | 22 | 18 | – |
| Age at diagnosis, median years (IQR) | 15.3 | 13.1 | 17.2 | .39 |
| (9.6–20.6) | (9.3–21.5) | (11.2–22.7) | ||
| Age at last follow-up, median years (IQR) | 21.2 | 19.6 | 22.4 | .56 |
| (14.8–29.0) | (15.2–28.01) | (14.0–30.6) | ||
| Race (% white) | 37 (92.5) | 20 (90.9) | 17 (94.4) | 1 |
| Ethnicity (% non-Hispanic) | 37 (92.5) | 20 (90.9) | 17 (94.4) | 1 |
| Karyotype | ||||
| 45,X | 30 (75.0) | 16 (72.7) | 14 (77.8) | 1 |
| Othera | 10 (25.0) | 6 (27.3) | 4 (22.2) | |
| Treatment with growth hormone | ||||
| Yes | 31 (77.5) | 17 (77.3) | 14 (77.8) | .97 |
| No | 9 (22.5) | 5 (22.7) | 4 (22.2) | |
| Treatment with estrogen (% yes) | 29 (72.5) | 17 (77.3) | 12 (66.7) | .5 |
| Hypertension (% yes) | 10 (25.0) | 9 (40.9) | 1 (5.6) | .01 |
| Less than 15 years of age (% yes) | 4 (10.0) | 4 (18.2) | 0 (0.0) | .11 |
| Bicuspid aortic valve (% yes) | 27 (67.5) | 16 (72.7) | 11 (61.1) | .44 |
| % right/left fusion | 20 (74.1) | 12 (75.0) | 8 (72.7) | .89 |
| Aortic stenosis (% yes) | 4 (10.0) | 2 (9.1) | 2 (11.1) | 1 |
| Aortic regurgitation (% yes) | 6 (15.0) | 3 (13.6) | 3 (16.7) | 1 |
| Coarctation of the aorta (% yes) | 8 (20.0) | 5 (22.7) | 3 (16.7) | .71 |
| Elongation of the transverse aorta | ||||
| Yes | 7 (17.5) | 3 (13.6) | 4 (22.2) | .48 |
| No/unknown | 33 (82.5) | 19 (86.3) | 14 (77.8) | |
| Other cardiac anomalies (% yes) | 19 (47.5) | 10 (45.5) | 9 (50.0) | .77 |
| Cardiac interventions other than those for coarctation of the aorta (N = 27 pts with cardiac anomalies; % yes) | 8 (29.6) | 4 (26.7) | 4 (33.3) | 1 |
- Note: All statistically significant values (p < .05) are provided in bold. Abbreviations: IQR, interquartile range; N, number of patients.
- a Other includes 45,X/46,XX (4 patients), 46,XiX(q10) (3 patients), mosaic 45,X/46,XY (1 patient), mosaic 46,Xdel(X)(p22.1) (1 patient), and unknown (1 patient).
The patients who were treated for their aortic dilatation were more likely to have hypertension at the time of diagnosis of aortic dilatation (41% of treated patients, 6% of untreated patients, p = .01). Of those with hypertension who were also less than 15 years of age at the time of aortic dilatation diagnosis, all four were treated with medications for aortic dilatation. There were no patients less than 15 years old with hypertension in the untreated group.
The presence of a bicuspid aortic valve was very common in this cohort (68%). Of the patients with a trisinuate aortic root, 14 patients had a bicuspid aortic valve with raphe between the right and left leaflets and four patients had a bicuspid aortic valve with raphe between the right and non-coronary leaflets. Nine patients had a bicuspid valve with a bisinuate root. One patient had a unicuspid and unicommissural aortic valve with two raphe and a trisinuate root.
Most of the patients had an aortic valve that functioned well, including those with a bicuspid aortic valve. Only 10% of patients had an aortic valve that was classified as having any stenosis (one with moderate stenosis and one with severe stenosis in the treatment group, and one with mild stenosis and one with moderate stenosis in the no treatment group). Aortic regurgitation was similar, and was present in 15% of patients overall (three with mild regurgitation in the treatment group, and one with mild regurgitation and two with moderate regurgitation in the untreated group).
Eight patients had a history of coarctation of the aorta (20%), with seven receiving surgical intervention and one receiving catheter-based intervention with subsequent re-dilatation. All of these patients with coarctation of the aorta had a bicuspid aortic valve as well. There were 27 patients with other associated cardiac anomalies (30%; Table S1). These anomalies were similarly seen in both the treated and untreated groups, and commonly included left superior vena cava, atrial and/or ventricular septal defect, and partial anomalous pulmonary venous return. Overall, 18% of the patients had elongation of the transverse aorta with similar distributions in the treatment (14%) and untreated (22%) groups.
Of those patients with aortic dilatation who were treated with medications for dilatation, the median age at treatment initiation was 18.8 years, with a range of 2.1 to 43 years (Table 2). The median duration of treatment during the study period was 2.8 years, with an interquartile range of 1.3 to 3.8 years. Half of the treatment group received atenolol as monotherapy, with five patients (23%) on losartan, three patients (14%) on a combination of losartan and atenolol, and the remainder of the patients on metoprolol, enalapril, or a combination of lisinopril and atenolol.
| Number of treated patients | 22 |
|---|---|
| Median length of treatment (IQR) | 2.8 years (1.3–3.8) |
| Median age at treatment initiation (IQR) | 18.8 years (2.1–43) |
| Medication type (N, %) | |
| Atenolol | 11 (50.0) |
| Losartan | 5 (22.7) |
| Losartan + Atenolol | 3 (13.6) |
| Metoprolol | 1 (4.5) |
| Enalapril | 1 (4.5) |
| Lisinopril + Atenolol | 1 (4.5) |
- Abbreviations: IQR, interquartile range; N, number of patients.
There was a significant difference in the unadjusted ascending aorta means between the treatment and the untreated group (Table 3 2.98 vs. 2.48 cm, p < .001). As there was no difference in body surface area between the groups, for those patients older than 15 years, this correlated with a significant difference in the unadjusted ascending aorta aortic size index (2.07 vs. 1.72 cm/m2, p < .001). This finding suggests a provider tendency to treat more severely affected patients, especially those patients who qualify by multiple dilatation indices (e.g., both absolute measurement and aortic size index). The mean absolute rate of ascending aorta dilatation following diagnosis for the entire cohort throughout the study period was 0.060 cm/year. For those patients in the treatment group, this rate increased to 0.062 cm/year. Patients in the untreated group had a statistically significantly lower rate of dilatation, 0.047 cm/year (p = .002), although this difference may not be of clinical significance.
| Cohort | Treated | Untreated | p-value | |
|---|---|---|---|---|
| N = 40 | N = 22 | N = 18 | ||
| Absolute ascending aorta means (cm, SE) | 2.98 (0.81) | 2.48 (0.53) | < .001 | |
| Body surface area (m2, SD) | 1.36 (0.41) | 1.33 (0.42) | .54 | |
| Ascending aortic size index (cm/m2, SD) | 2.34 (0.80) | 2.01 (0.57) | .002 | |
| Subjects older than 15 years (cm/m2, SD) | 2.07 (0.40) | 1.72 (0.33) | < .001 | |
| Rate of dilatation (cm/yr, SE) | 0.060 (0.006) | 0.062 (0.010) | 0.047 (0.008) | .002 |
- Note: All statistically significant values (p < .05) are provided in bold.
- Abbreviations: cm, centimeter; m2, square meters; N, number of patients; SD, standard deviation; SE, standard error; yr, year.
In the adjusted model, there was no difference seen in either the mean absolute aortic root measurements (Table 4, p = .13) or the mean residual aortic root measurements of the treatment and untreated groups (p = .95). There was also no difference in the change of the residual aortic root measurements over time since baseline (p = .46) or following the initiation of medications (p = .99). In the ascending aorta adjusted predictive model, there again was a difference seen between both the absolute measurements (2.753 vs. 2.324 cm, p < .001) and the residual measurements (0.156 vs. 0.030 cm, p < .001) in the treatment and untreated groups. Over time, those patients with larger ascending aortas remain dilated. However, between the treatment and untreated groups, there was no difference in the change of the residual ascending aorta measurements over time since baseline (p = .37) or following the initiation of medications (p = .54).
| Treated | Untreated | Effect estimatea | p-value | |
|---|---|---|---|---|
| N = 22 | N = 18 | |||
| Aortic root adjusted model | ||||
| Mean absolute measurement (cm, SE) | 2.709 (0.046) | 2.625 (0.045) | - | .13 |
| Mean residual difference (cm, SE) | −0.005 (0.024) | −0.002 (0.030) | −0.001 | .95 |
| Change over time since baseline | −0.005 | .46 | ||
| Change over time since medication initiation | −0.0001 | .99 | ||
| Ascending aorta adjusted model | ||||
| Mean absolute measurement (cm, SE) | 2.753 (0.067) | 2.324 (0.065) | - | < .001 |
| Mean residual difference (cm, SE) | 0.156 (0.029) | 0.030 (0.023) | −0.063 | < .001 |
| Change over time since baseline | 0.006 | .37 | ||
| Change over time since medication initiation | −0.004 | .54 |
- Note: All statistically significant values (p < .05) are provided in bold.
- Abbreviations: cm, centimeter; N, number of patients; SE, standard error.
- a Effect estimate refers to comparison between treatment and no treatment groups, with no treatment group as reference; model adjusted for age, age squared, body surface area, imaging modality, and presence of risk factors for dilatation in Turner syndrome patients: bicuspid aortic valve, hypertension, and coarctation of the aorta.
To assess whether these results were affected by younger patients whose aortic dilatation may normalize as their body surface area changed due to normal growth or older patients with more progressive dilatation, the ascending aorta analysis was repeated with subgroupings of patients based on ages of 15, 21, 30, and 40 years (Table S2). In this exploratory sub-analysis, there was no significant difference in findings. There was a trend toward a negative effect if not treated, with the ascending aortic change effect measurements over time following medication initiation becoming progressively more negative, but this only became statistically significant in the very small number of patients older than 40 years (N = 11; effect −0.069, p = .006).
4 DISCUSSION
In a single center cohort of Turner syndrome patients with aortic dilatation, the rate of aortic dilatation was slightly higher than seen for previous cohorts of Turner syndrome patients, suggesting a more aggressive phenotype in those with aortic dilatation. Prior to the implementation of expert consensus guidelines, patients were more likely to be treated with medications if they had hypertension at the time of diagnosis and if they met diagnostic criteria for dilatation by multiple indices. Although there are a number of important limitations to the finding, there was no significant difference in aortic growth over time between those patients treated and untreated for dilatation, after adjusting for common aortic dilatation risk factors such as the presence of a bicuspid aortic valve.
This study cohort was selected from one of the largest pediatric Turner syndrome centers in the United States, which likely contributes to the earlier age at diagnosis of aortic dilatation than seen in other Turner syndrome descriptive reports (Donadille et al., 2012; Duijnhouwer et al., 2018). An EMR query enabled comprehensive patient identification of potential subjects. Most patients were followed by a pediatric cardiology team specializing in cardiovascular genetics, with the remainder followed by general pediatric cardiologists. Although providers had similar approaches, there was heterogeneity in monitoring and treatment practices from the time of the earliest diagnosis in the cohort (2004) until the introduction of robust expert consensus guidelines (2017) (Gravholt et al., 2017). Prior to guideline publication, it is likely that medication treatment decisions were based on the provider's own judgment weighing the severity and rate of aortic dilatation in the setting of risk factors for progressive aortic dilatation. Patients with hypertension at the time of aortic dilatation diagnosis were disproportionally more likely to be treated with medications for their dilatation, likely due to these overlapping therapeutic indications. Nearly half of these patients with hypertension in the treatment group were less than 15 years old, suggesting provider concern for progressive dilatation leading to more aggressive management in these younger patients. The implementation of clinical practice guidelines will likely impact the monitoring and treatment strategies of this cohort in the future, and would benefit from prospective study.
Many of the characteristic features associated with Turner syndrome, such as monosomy X, treatment with growth hormone and estrogen, and risk factors for aortic dilatation such as coarctation of the aorta and hypertension are similar to those previously reported (Bondy, 2008; Donadille et al., 2012; Duijnhouwer et al., 2018; Lopez et al., 2008). However, in this cohort of Turner syndrome subjects with aortic dilatation, the presence of a bicuspid aortic valve was more common than typically reported (67.5 vs. 14–30%) (Bondy, 2008; Donadille et al., 2012; Duijnhouwer et al., 2018; Lin, Santoro, High, Goldenberg, & Gutmark-Little, 2020; Lopez et al., 2008), which likely contributes to the significant rate of aortic dilation observed. The prevalence of bicuspid aortic valve in all Turner syndrome patients followed at this center, including patients without aortic dilatation, is much lower (39%) (Kim et al., 2011), and consistent with the rates seen in the general Turner syndrome population. Other studies have shown that Turner syndrome patients with a bicuspid aortic valve have significantly larger aortas than Turner syndrome patients with a trileaflet valve (Hjerrild et al., 2010), similar to findings seen in the general population (Abdulkareem, Smelt, & Jahangiri, 2013). Of note, the recent development of normative equations by Quezada et al to calculate Turner-specific Z-score excluded the 25.2% of their cohort with a bicuspid aortic valve (Quezada et al., 2015). This index has been recommended for use in monitoring aortic dilatation in all Turner syndrome patients under 15 years of age, but given the impact of a bicuspid aortic valve on dilatation, additional consideration may be needed when evaluating young Turner syndrome patients with a bicuspid aortic valve.
The rate of ascending aortic growth in this population of Turner syndrome patients with known aortic dilatation was slightly higher than the rates previously reported in cohorts of Turner syndrome patients that include those both with and without aortic dilatation. Other models have suggested a rate of ascending aortic growth from 0.20 to 0.24 mm/year (Duijnhouwer et al., 2018; Mortensen et al., 2011). The rate of 0.6 mm/year seen in this cohort during the study period suggests that some Turner syndrome patients are at risk for aortic dilatation that is more progressive than the general Turner syndrome population. Some of growth velocity may be multifactorial in etiology, including bicuspid valve aortopathy. Once identified as having aortic dilatation, Turner syndrome patients should be carefully monitored.
Subjects with greater degrees of ascending aortic dilatation were more likely to be treated for aortic dilatation, both at baseline and over time. If they met diagnostic criteria for dilatation both by absolute value and by aortic size index, patients were also more likely to be treated, suggesting that providers were influenced by multiple supporting metrics. As with the hypertension findings above, it is likely that imaging/monitoring frequency and treatment differences may standardize now that there are clear expert consensus guidelines for medical management (Gravholt et al., 2017; Silberbach et al., 2018).
Current medical management recommendations for Turner syndrome patients with aortic dilatation at increased risk of dissection is based on consensus opinion (Gravholt et al., 2017; Silberbach et al., 2018). In this small cohort prior to the initiation of the guidelines, the rate of change in aortic measures was not consistently associated with medication use although this finding is subject to a number of important limitations. There was a significant difference in the unadjusted ascending aorta means between the treated and the untreated groups, but this difference was not sustained within the adjusted model. Such a model reflects expected growth in aortic dimensions over time, but may also introduce confounders that influence the final results. Additionally, the treated subjects in this cohort tended to have more dilated aortas to begin with, and therefore, potentially have a more aggressive phenotype. Treatment randomization may have been necessary to see an effect of medications on the rate of progression of aortic dilatation in this group. Finally, a median treatment period of 2.8 years may not have been a sufficient amount of time to assess medication effect. Ultimately, while not definitively conclusive, these findings are an important first step in the understanding of the management of TS aortic dilatation and highlight the need for larger, multicenter, randomized trials to assess the effect of medications on the rate of aortic dilatation.
Importantly, aortic dissection has been seen in Turner syndrome patients without a significant change in the rate of aortic growth (Carlson et al., 2012), suggesting that there may be other factors contributing to the risk of dissection such as aortic wall composition (Carlson & Silberbach, 2007; Grewal et al., 2020). Medications may have beneficial effects on these other factors that are not quantified by the rate of change in aortic measures. Additional studies are also needed to determine if medications reduce the risk of aortic dissection in Turner syndrome patients, which this study was not sufficiently powered to assess.
4.1 Limitations
This study is limited by the small, retrospective, single-center nature of the cohort. There is likely a referral and selection bias in the cohort, as the subjects were referred for Turner syndrome care, and therefore may represent those with more significant disease, including those with a variety of additional cardiovascular lesions. During the study period, which was before the implementation of Turner syndrome-specific screening and diagnostic recommendations, there was heterogeneity of provider practice patterns with regard to management, follow-up, and treatment strategies. This may also have introduced an ascertainment bias, particularly in the patients who were treated for aortic dilatation. Furthermore, inclusion only of patients who meet criteria for aortic dilatation under current definitions may exclude some Turner syndrome patients who were treated for less significant dilatation. This limitation is likely more pronounced in younger patients as the Turner-specific Z-score generates lower Z-scores than previously-used metrics. Finally, the use of overlapping echocardiography and MRI data likely introduces measurement variability in the results.
Although this study provides important information about clinical outcomes following initiation of medication for aortic dilatation, there are also several important caveats. Blood pressure control in hypertensive subjects was not able to be assessed well due to the retrospective nature of this study. Furthermore, while the longitudinal nature of this study permitted evaluating medication use as a time varying covariate, it is important to recognize our modeling may not have fully characterized changes in aortic dilatation following medication initiation. It is suspected that the higher ascending aorta growth rate seen in the treatment group is due to more aggressive phenotypes that influenced treatment decisions in this group, but medications may have a deleterious effect that this study was not powered to assess. Additionally, due to the small number of treated subjects, an exploratory sub-analysis for efficacy of specific medication classes (beta-blocker, angiotensin receptor blocker, or angiotensin-converting enzyme inhibitor) was unable to be performed.
Ascending aorta dilatation is clearly impacted by age (Corbitt, Maslen, Prakash, Morris, & Silberbach, 2018; Duijnhouwer et al., 2018; Olsson, Thelin, Ståhle, Ekbom, & Granath, 2006), and the development of dissection generally is not seen until the fourth decade of life. The relatively young age of this cohort at diagnosis and follow-up time period may not have allowed for significant enough aortic growth to detect a treatment difference. This is supported by the trend toward a negative effect seen in the treatment groups of older patients, but needs to be evaluated in a larger population. Finally, this study was not adequately powered to assess morbidity and mortality outcome differences between treatment groups.
5 CONCLUSION
In this analysis of Turner syndrome patients with diagnosed aortic dilatation prior to the implementation of expert consensus guidelines, these patients were more likely to be treated with medications if they had hypertension at the time of diagnosis, and if they met diagnostic criteria by multiple indices. In this small cohort, the rate of aortic dilatation was modest among those on medications. Further study is needed to establish the efficacy of medical therapy on the progression of Turner syndrome-related aortic dilatation.
ACKNOWLEDGMENTS
We thank the patients and families of the Turner Syndrome Center at Cincinnati Children's Hospital Medical Center.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Colleen Pater, Nicole Brown, and Iris Gutmark-Little conceived of the presented idea, designed the research study, and drafted the manuscript. Colleen Pater and Justin Tretter performed the data collection. Lisa Martin and Colleen Pater performed the data analysis. Phillipe Backeljauw, Lisa Martin, and Justin Tretter provided critical feedback in the study design and analyses. All authors discussed the results and contributed to the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




