The role of novel COQ8B mutations in glomerulopathy and related kidney defects
Funding information: National Institute of General Medical Sciences, Grant/Award Number: R00GM11421; National Institute of Health, Grant/Award Number: S10 OD011981
Abstract
Background and Objectives
Glomerulopathies affect kidney glomeruli and can lead to end-stage renal disease if untreated. Clinical and experimental evidence have identified numerous (>20) genetic mutations in the mitochondrial coenzyme Q8B protein (COQ8B) primarily associated with nephrotic syndrome. Yet, little else is understood about COQ8B activity in renal pathogenesis and its role in mitochondrial dysfunction. We identified additional novel COQ8B mutations in a glomerulopathy patient and aimed to define the potential structural and functional defects of COQ8B mutations.
Design, Setting, Participants, and Measurements
Whole exome sequencing was performed on a Hispanic female presenting with proteinuria. Novel mutations in the COQ8B gene were identified. The effects of mutation on protein function, mitochondrial morphology, and disease progression were investigated by histopathology, transmission electron microscopy, homology modeling, and in silico structural analysis.
Results
We have characterized the pathophysiology of novel COQ8B mutations, compound heterozygous for two alterations c.1037T>G (p.I346S), and c.1560G>A (p.W520X), in the progression of proteinuria in a Hispanic female. Histopathology revealed defects in podocyte structure and mitochondrial morphology. In silico and computation analyses highlight possible structural origins of COQ8B dysfunction in the presence of mutations.
Conclusions
Novel mutations in COQ8B present promising biomarkers for the early detection and therapeutic targeting of mitochondrial glomerulopathy. Insights from structural modeling suggest roles of mutation-dependent alterations in COQ8B allosteric regulation, protein folding, or stability in renal pathogenesis.
1 INTRODUCTION
Many chronic kidney diseases and nephrotic syndromes have recently been shown to result from dysfunction in mitochondrial proteins stemming from genetic mutations (Emma, Montini, Parikh, & Salviati, 2016; Finsterer & Scorza, 2017). Patients with nephrotic syndrome often present with proteinuria, hypoalbuminemia, edema, resistant to treatment, and often succumb to end-stage renal disease (ESRD) (Ashraf et al., 2013; Korkmaz et al., 2016; Atmaca et al., 2017). Renal biopsy most often reveals focal segmental glomerulosclerosis (FSGS). Identification of the biochemical functions of mutant proteins involved in mitochondrial renal disorders is essential for understanding the mechanism of disease and for developing a targeted therapy to prevent these patients from renal failure.
Among the more prevalent underlying causes of mitochondrial-related renal disorders is the deficiency in Coenzyme Q (CoQ) biosynthesis. CoQ is a redox-active lipid present in all cell membranes including the inner mitochondrial membrane and is an essential cofactor in electron transport in the mitochondrial respiratory chain. Numerous reports on the broader cellular functions within and outside of the mitochondria highlight the complex and diverse role of CoQ on human health (Stefely & Pagliarini, 2017). To date, the biosynthesis of CoQ and its biochemical activities are still not fully understood.
Studies in yeast and humans have revealed the importance of a multi-component biosynthetic complex underlying the synthesis of CoQ. Loss of function or expression of any individual component can lead to errors in the biosynthetic process and the development of diseases affecting various organ systems. While some genetic mutations in genes associated with CoQ production can be broadly affecting, more acute effects are observed in the presence of mutations in genes important to the terminal phase of CoQ production (Stefely & Pagliarini, 2017). These include COQ8A (Gene Accession Number: NM_020247.5) and COQ8B (Gene Accession Number: NM_024876.4) encoding the aarF domain-containing kinase proteins COQ8A (previously termed ADCK3) and COQ8B (previously termed ADCK4), respectively. In COQ8B specifically, more than 26 mutations have been reported in the Human Gene Mutation Database (HGMD), including biallelic variants that cause autosomal recessive nephrotic syndromes. Such mutations appear to have a predilection for disrupting kidney function by a yet to be defined mechanism (Ashraf et al., 2013; Vazquez Fonseca et al., 2018; Yang, Yang, & Hu, 2018). While expressed in the mitochondria of podocytes, proximal tubules, and collecting ducts, the functional role of COQ8B in renal homeostasis is unclear.
Limited characterizations of the biological function of the COQ8B kinase in CoQ biosynthesis and other related mitochondrial functions, as well as the spectrum of mutations that interfere with these activities, hinder progress towards understanding the role of COQ8B in the progression of glomerulopathies necessary for advancing the development of new treatments. Herein, we present the pathogenesis and describe potential mechanisms of action of newly identified COQ8B mutations in a patient with glomerulopathy.
2 RESULTS
2.1 Case presentation
Here, we report a 20-year old Hispanic female who initially presented at the age of 5 years with proteinuria following routine screening. At presentation, her urinary protein/creatinine ratio (uPCR) was elevated (uPCR 0.5 g/g, reference range < 0.2 g/g). She had no edema, hematuria, fever, or any other concerning symptoms. The patient's medical history prior to presentation was unremarkable. Her physical examination did not reveal any dysmorphic features. Blood pressure was within normal limits and the patient had essentially normal kidney function.
The patient's proteinuria and kidney functions were noted to have worsened slowly over time. Lisinopril and Losartan were started at 6 years of age but had no marked effect. Her first renal biopsy was conducted at 8 years of age and did not reveal any specific pathology (see below for details). Her second renal biopsy at age 11 years revealed focal and global segmental glomerulosclerosis described as secondary type. Her creatinine levels were noted as >1 mg/dl (reference range 0.4–0.9 mg/dl) by 15 years of age and had doubled by 18 years of age (3.8 mg/dl, reference range 0.4–1.2 mg/dl). Serum albumin levels range 2.5–3.5 g/dl (reference range 3.6–5.1 g/dl). Blood pressure was within normal limits until kidney functions further deteriorated at 17 years old.
Overt symptoms of edema/nephrotic syndrome were not observed until 1 year prior to ESRD. Notably, there was no prior history of steroid use or immunosuppressive medications. Renal ultrasonography performed at age 16 years revealed bilateral medullary nephrocalcinosis and grade 1 hydronephrosis (Supplementary Figure 1). Interestingly, she had 3 episodes of rhabdomyolysis, at age 16 years, 17 years, and 18 years (Creatine kinase peak of 10,411–11,883 U/L in these episodes, reference range 21–215 U/L). Whole-exome sequencing performed at the age of 18 years revealed COQ8B mutations. The patient was started on 20 mg/kg coenzyme Q10 (2,100 mg daily) at the age of 18 years, which was well-tolerated, but unfortunately, she developed ESRD a year later, and peritoneal dialysis was started.
2.2 Family history
The patient's extensive family history of renal disorders includes congenital anomalies of the urogenital tract in her siblings and on her paternal side (Supplementary Figure 2). Her father, currently 49 years of age, was diagnosed with proteinuria during a health screen at the age of 34. A kidney biopsy in light of worsening proteinuria at the age of 38 years revealed membranous glomerulonephritis per the report. Unfortunately, the kidney biopsy was unavailable to review due to medical facility guidelines in which biopsies are discarded after 10 years. His most recent creatinine was reported as 1.1 mg/dl. Interestingly, both the father and his brother have atypical collecting system morphology. The father has a bilateral duplicated collecting system and his brother (currently 51 years old) has a unilateral duplicated collecting system. The patient's mother, age 51 years old, has no known renal abnormalities, but was recently diagnosed with inflammatory bowel disease (Crohn's disease). The parents deny consanguinity. The patient's siblings have demonstrated structural congenital renal anomalies. Her 22-year-old brother has had a history of left hydronephrosis during infancy, which resolved at 1 year of age. Later, he was diagnosed with right ureteropelvic junction obstruction (UPJO) at 15 years of age, which required a pyeloplasty. He now has hypertension with left ventricular hypertrophy, and his creatinine is 1.1 mg/dl (reference range 0.6–1.2 mg/dl). The patient's younger sister, 18 years of age, has a unilateral duplicated collecting system, but normal renal functions and creatinine levels (0.8 mg/dl, reference range 0.4–1.2 mg/dl).
2.3 Histopathology and electron microscopy
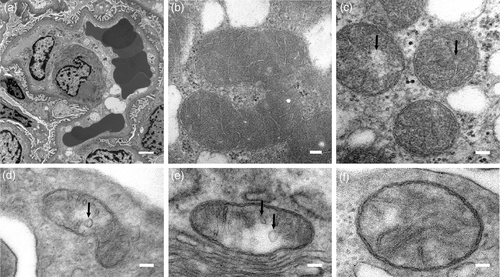
Following worsening proteinuria (uPCR levels of 1 g/g), the patient's first kidney biopsy revealed focal basement membrane abnormalities. Collagen IV staining was normal. Electron microscopy (EM) revealed variable looking glomerular basement membrane (GBM), with some loops showing normal GBM while in other loops the GBM thickness was irregular and characterized by heterogeneous lucency of the lamina densa and lamina interna rara. Foot processes were preserved (Figure 1(a)). Unfortunately, mitochondria were not examined at initial biopsy. Reexamination of the kidney tissue sections for better characterization of mitochondrial morphology and other ultra-structures showed dysmorphic mitochondria, cristae disarrangement in the podocytes, ballooning of cristae, and loss of cristae formation (Figure 1(b)-(f)). The second kidney biopsy at age 11 years revealed focal segmental and focal global glomerulosclerosis of secondary type indicated by the preservation of the glomerular podocyte foot processes. Mild patchy interstitial fibrosis and tubular atrophy were also noted.
2.4 Genetic analysis
Given the decline of the patient's renal functions and worsening proteinuria, whole exome sequencing was obtained. Bioinformatics analysis and filtering of the proband, the mother, father, and siblings revealed alterations with clinical relevance. Two novel compound heterozygous mutations were identified in the COQ8B gene (Gene Accession Number: NM_024876.4) (Figure 2). The first mutation, COQ8B c.1037T>G (p.I346S), is a genetic alteration located in coding exon 12 of COQ8B. This alteration results in mutation of a highly conserved isoleucine (I) to serine (S) mutation at amino acid position 346 (I346S) (Supplementary Figure 3a,b). The COQ8B c.1037T>G alternation was not observed among 6,503 individuals tested. Allele frequency data for this nucleotide position are currently unavailable. This p.1346S alteration is predicted to be damaging by Polyphen (score of 0.98) and deleterious by SIFT in silico analyses (score of zero).
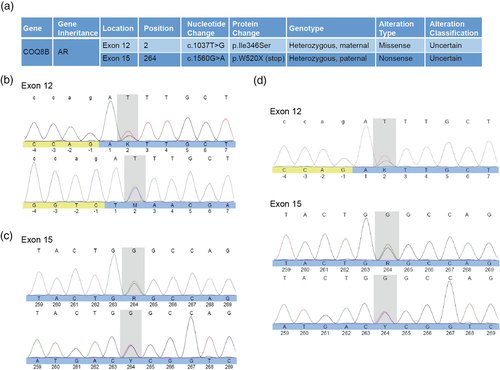
The second mutation was identified as COQ8B c.1560G>A (p.W520X), located in coding exon 14 of COQ8B gene, resulting in a sequence truncation at residue 519 of 544 encoded amino acids. Such nonsense mutations occurring in codons of the last exon, or within 50–55 nucleotides upstream of the 3′ most exon-exon junction, usually fail to elicit nonsense-mediated decay (Maquat, 2004). Therefore, a truncated COQ8B protein could be expressed. Based on data from the NHLBI Exome Sequencing project (ESP), the COQ8B c.1560G>A alteration is ultra-rare, with only 1 observation among 12,998 total alleles studied (<0.001%). Data from the Exome Aggregation Consortium (ExAC) reveal observation of the c.1560G>A (W520X) alteration in <0.006% (4/71,076) of total alleles studied, with <0.008% (3/39,422) European alleles and < 0.018% (1/5,428) Latino alleles.
Co-segregation analysis revealed that the patient and her father are both heterozygous for the W520X mutation. The patient and mother are both heterozygous for the I346S mutation. Specific mutation analysis in both of the patient's siblings revealed that her sister carries the W520X truncation mutation while the brother is negative for both mutations (data not shown). It is unclear if the structural anomaly observed in the sister (duplicated collecting system) is associated with COQ8B mutation.
2.5 Structural analysis of COQ8B mutation
The mutations discovered in this report map to the protein kinase-like (PKL) domain of COQ8B for which there are currently no published structures. A structure of this domain in the related UbiB family member, COQ8A, was recently determined, providing a basis for understanding the effect of disease mutations in COQ8B (Stefely et al., 2015). Notably, COQ8B contains an 18 amino acid extension at its C-terminus proximal to the W520 termination site (Figure 3(a)). The C-terminus in UbiB proteins is also the least conserved region of the PKL domain, whereas I346 is absolutely conserved across evolution (Supplementary Figure 3a). Based on sequence alone, mutation at W520 may be specific to COQ8B function, whereas mutation at I346 may influence a universal function or property of UbiB family members.
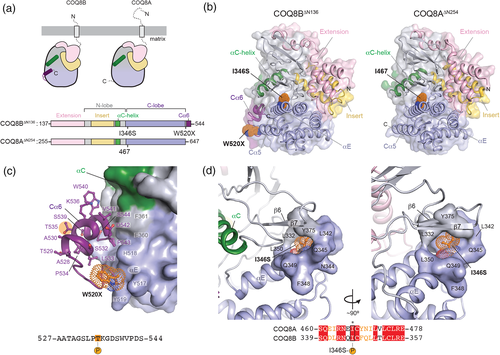
To evaluate the effects of mutation on COQ8B-specific functions, we generated a homology model of the COQ8B PKL domain (residues 137–544: COQ8B∆N136) with I-TASSER (Yang et al., 2014) using the structure of COQ8A∆N255 (PDB ID: 4PED; residues 255–644) as a reference (Stefely et al., 2015). Both COQ8B∆N136 and COQ8A∆N255 adopt a common protein kinase fold consisting of an N-lobe and C-lobe (Figure 3(b)). The C-terminal extension of COQ8B∆N136 was visualized in our homology model and adopts an alpha helical structure matching secondary structure predictions (Pei & Grishin, 2007). COQ8B W520 directly precedes the predicted C-terminal helix, which we have termed Cα6. Residues of the C-lobe and αC helix stabilize interactions with the Cα6 helix (Figure 3(c)). In this orientation, truncation of COQ8B at W520 is not expected to unfold COQ8B. However, the large interaction surface between Cα6 and the C-lobe in our model may be important for inter- or intra-molecular protein interactions necessary for the allosteric regulation of COQ8B function. Analysis of wild-type COQ8B using the NetPhos3.1 server (Blom, Sicheritz-Pontén, Gupta, Gammeltoft, & Brunak, 2004) reveals two putative consensus sites for serine (S) and threonine (T) phosphorylation in the Cα6 helix, one of which (T535) scores highly (0.73/1.00) for PKC-mediated phosphorylation, although at present a link between COQ8B and PKC has not been established.
The absolute conservation of I346 in UbiB family proteins throughout evolution suggests a potentially universal biological importance of the residue. In the wild-type COQ8B∆N136 model, I346 is buried in a hydrophobic pocket resembling substrate binding clefts in protein kinases (Figure 3(d)). This architecture is conserved in COQ8A, but it is unclear from these structures how mutation to serine could affect kinase function in either COQ8A or COQ8B. One possibility is the presence of a negatively charged serine residue prevents important substrate interactions. Alternatively, proximity to the hydroxyl group of Y375 may result in electrostatic repulsions that disrupt the hydrophobic pocket and potentially destabilize the COQ8B fold. The I346S containing sequence is also predicted to be a strong consensus site for phosphorylation by PKA (0.81/1.00), which may also alter necessary regulation of COQ8B (Figure 3(d)).
3 DISCUSSION
Chronic kidney diseases (CKD) are among the most prevalent human health conditions facing society today. Nephrotic syndrome (NS), a subset of CKD affecting the glomeruli, is the most common CKD in adults and children, and is characterized by proteinuria, hypoalbuminemia, edema, and hyperlipidemia. Nearly 80% of pediatric cases of NS are steroid-sensitive nephrotic syndrome (SSNS), and 20% are steroid-resistant nephrotic syndrome (SRNS). Although several genes have been implicated in NS, no improvement in treatment options or outcomes in these patients have yet been noted. Nonetheless, early diagnostics have been shown to play an impactful role in the detection and treatment of CKDs including autosomal recessive COQ8B -glomerulopathy (COQ8B -GN).
Nephrotic syndromes like COQ8B-GN are a form of mitochondrial nephropathy caused by defective proteins implicated in the biosynthesis of the critical respiratory molecule, CoQ. The onset of COQ8B-GN symptoms occur typically between 5–14 years of age, with the majority of patients developing ESRD in the second decade of life (Ashraf et al., 2013; Atmaca et al., 2017; Korkmaz et al., 2016). In our patient, onset of disease was noted at the age of 5 years and diagnosed with ESRD at age of 17 years. Diagnosis of the underlying etiology of proteinuria is a challenge for nephrologists, especially in mild cases when the disease has not yet progressed, even if kidney biopsy is obtained. The discovery of proteinuria is usually incidental, and some patients do not develop overt nephrotic syndrome or until the second decade of life, as in our patient. Her renal histology was more consistent with focal segmental glomerulosclerosis (FSGS), a common pathological lesion of SRNS indicative of a significant risk of rapid progression to ESRD. However, renal histology can vary from nonspecific (early) FSGS or collapsing FSGS as the disease progresses (Ashraf et al., 2013; Desbats, Lunardi, Doimo, Trevisson, & Salviati, 2014; Korkmaz et al., 2016; Park et al., 2017). Recent advances in exome sequencing technology have had a tremendous impact in furthering our understanding of NS-related glomerulopathies. Genetic testing is crucial for timely diagnosis of patients with early-onset proteinuria, which will not only progress our understanding of the genetic basis of kidney disease and facilitate early interventions, but also prevent unnecessary invasive procedures and immunosuppressive medications.
The current therapy for glomerulopathies arising from mutation-related deficiencies in CoQ biosynthesis is high dose oral supplementation of CoQ10, for which there is some evidence of clinical improvement in renal function (Ashraf et al., 2013; Atmaca et al., 2017; Eroglu et al., 2018). In one such study, eight patients with asymptomatic proteinuria showed significantly decreased proteinuria (follow up duration, median 11.5 mo, range 4–21 mo) with no changes in eGFR before and after CoQ10 initiation (Eroglu et al., 2018). Despite these reports, it is unclear if CoQ10 therapy was sufficiently bioavailable to prevent podocyte dysfunction, nor were we able to evaluate its efficacy in our patient. CoQ10 was administered at age 18 years, after the pathology of her disease had progressed.
COQ8A and COQ8B are two of 13 different genes required for CoQ10 biosynthesis. Mutations in these genes have been associated with CoQ10 deficiency presenting with a variety of clinical manifestations. Although COQ8B is paralogous to COQ8A, COQ8A mutations cause neurological disorders in children, whereas patients with COQ8B mutations tend to have a renal-limited phenotype and are rarely associated with neurological manifestations (Lolin et al., 2017). Extra-renal manifestations of COQ8B mutations reported include developmental delay, intellectual disability, autism, seizures, retinitis pigmentosa, dilated cardiomyopathy, hypertrophic cardiology, pericardial effusion, pulmonary hypertension, short stature, hypothyroidism, and ear malformation. An isolated published report associates Crohn's disease with COQ8B glomerulopathy (Kakiuchi et al., 2019). It may be that there is either another nonexome regulatory gene which is altered in the family of our patient's father who also has Chron's disease, or these features are multifactorial in his family. A whole genome sequence might be helpful in delineating this further.
Although the exact mechanisms by which previously reported COQ8B mutations facilitate renal dysfunction are still unknown, it has been proposed that disease symptoms may be related to reduced migration rates in podocytes (Ashraf et al., 2013; Stefely & Pagliarini, 2017). Defining the biological function of COQ8B protein in podocytes is crucial to advancing therapeutic options in the treatment of COQ8B nephropathy. Additionally, investigations into the role of COQ8B in mitochondrial signaling will further our molecular understanding of the role of COQ8B in such kidney diseases. The UbiB family of proteins are single transmembrane spanning kinase-like proteins with active site elements hypothetically capable of catalyzing ATP-dependent substrate phosphorylation for signal transduction in cellular proliferation, gene expression, metabolism, migration, membrane transport, and other functions (Kannan, Taylor, Zhai, Venter, & Manning, 2007). Although it is unclear what the catalytic properties of COQ8B are, studies in COQ8A reveal catalytic autoinhibition that may be overcome by some secondary mechanism, such as posttranslational modification or dimerization (Stefely et al., 2015; Khadria et al., 2014). Notably, COQ8A has been shown to rescue CoQ substrate phosphorylation in yeast (Xie et al., 2011). In COQ8B, phosphorylation at I346S may alter the catalytic output of COQ8B. The presence of not one, but two mutations in our patient that could potentially change the landscape of posttranslational modifications in COQ8B suggests that a breakdown in higher-order regulation may be involved in the pathology of COQ8B -related glomerulopathy. While our homology model provides fascinating insight into the molecular basis of our patient's syndrome, biochemical and structural investigations into COQ8B will be instrumental in overcoming barriers to understanding its role in nephrotic disease.
4 CONCLUSION
COQ8B glomerulopathy should be suspected in patients with proteinuria in the first decade of life. Early genetic testing for asymptomatic proteinuria is crucial for the detection of potential treatable causes, especially in patients with a family history of significant renal pathology. Previously identified COQ8B mutations are mostly renal-limited type. Here we report novel COQ8B mutations in a patient that presented with proteinuria related to FSGS that progressed to ESRD. Structural analysis suggests that mutations may impact COQ8B function through alterations in both catalytic activity and allosteric regulation of the protein. However, additional studies are needed to understand the molecular basis of COQ8B mutation in such glomerulopathies and subsequently, the discovery of targeted therapies.
5 MATERIALS AND METHODS
5.1 Genetic analysis
The COQ8B gene contains 14 coding exons and one noncoding region located on chromosome 19q13.1. Full exome sequencing, bioinformatics analysis, filtering of proband, mother and father revealed alterations with clinical relevance in COQ8B. Specific gene site analysis for these mutations were obtained for the siblings.
5.2 Structural modeling of COQ8B kinase domain mutations
The structure of the kinase domain of COQ8A (PDB ID: 4PED, (Stefely et al., 2015)) was used as a template to model the kinase domain structure of COQ8B (residues 137 to 544) encoded by COQ8B (Gene Accession Number: NM_024876.4) in I-TASSER (Yang et al., 2014). The resultant model of the wild-type COQ8B structure had a positive C-score of 0.09 and TM-score of 0.73 ± 11, consistent with reliable model I-TASSER criteria (Roy, Kucukural, & Zhang, 2010). The secondary structure of elements in the kinase domain of COQ8B absent in the COQ8A structure were cross-checked against secondary structure predicted by JPRED (Drozdetskiy, Cole, Procter, & Barton, 2015) and ProMALS (Pei & Grishin, 2007). The model in the presence of mutations W520X and I346S were evaluated in PyMOL v2.3 (Schrodinger, 2015).
ACKNOWLEDGEMENTS
We thank the patient and their family for their cooperation during our study. We thank Dr. William A. Day for assistance with electron microscopy performed in the University of Arizona Life Sciences North Imaging Facility supported by funding proved by the NIH grant number S10 OD011981. This work was also supported by a grant from the National Institute of General Medical Sciences (R00GM11421) to Thomas M. Tomasiak.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Asmaa S. AbuMaziad, Chyi Chyi Chong, Maureen K. Galindo, and H. Eugene Hoyme gathered detailed clinical information and performed genetic analyses. Tarjani M. Thaker and Thomas M. Tomasiak performed protein modeling and mutation analysis. Asmaa S. AbuMaziad, Tarjani M. Thaker, Thomas M. Tomasiak, Chyi Chyi Chong, Maureen K. Galindo, and H Eugene Hoyme critically reviewed the paper. Asmaa S. AbuMaziad and Tarjani M. Thaker analyzed the data and prepared the original and revised manuscript for submission.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




