Pathogenic variants in EP300 and ANKRD11 in patients with phenotypes overlapping Cornelia de Lange syndrome
Funding information: Diputación General de Aragón, Grant/Award Number: B32_17R; Fondazione Pisa, Grant/Award Number: 120/16; Spanish Ministry of Health - Fondo de Investigación Sanitaria, Grant/Award Number: PI19/01860
Abstract
Cornelia de Lange syndrome (CdLS), Rubinstein–Taybi syndrome (RSTS), and KBG syndrome are three distinct developmental human disorders. Variants in seven genes belonging to the cohesin pathway, NIPBL, SMC1A, SMC3, HDAC8, RAD21, ANKRD11, and BRD4, were identified in about 80% of patients with CdLS, suggesting that additional causative genes remain to be discovered. Two genes, CREBBP and EP300, have been associated with RSTS, whereas KBG results from variants in ANKRD11. By exome sequencing, a genetic cause was elucidated in two patients with clinical diagnosis of CdLS but without variants in known CdLS genes. In particular, genetic variants in EP300 and ANKRD11 were identified in the two patients with CdLS. EP300 and ANKRD11 pathogenic variants caused the reduction of the respective proteins suggesting that their low levels contribute to CdLS-like phenotype. These findings highlight the clinical overlap between CdLS, RSTS, and KBG and support the notion that these rare disorders are linked to abnormal chromatin remodeling, which in turn affects the transcriptional machinery.
1 INTRODUCTION
Cornelia de Lange syndrome (CdLS, MIM#s 122470, 300590, 610759, 614701, 300882) is a rare developmental disease characterized by pre-natal and post-natal growth retardation, facial dysmorphism, cognitive impairment, and structural defects in multiple organs (Mannini, Cucco, Quarantotti, Krantz, & Musio, 2013). This wide phenotypic variability of CdLS depends on its wide genetic heterogeneity. In fact, seven CdLS-causative genes (NIPBL, SMC1A, SMC3, HDAC8, RAD21, BRD4, and ANKRD11) have been identified up to now (Kline et al., 2018). All these genes encode core cohesin components or associated factors. The cohesin complex plays multiple roles in biological processes such as sister chromatid cohesion, gene expression regulation, and DNA repair (Cucco & Musio, 2016; Merkenschlager & Odom, 2013; Musio, 2020). The importance of cohesin in maintaining genome stability is proven by the identification of somatic cohesin variants in many human cancers (Kon et al., 2013; Sarogni et al., 2019).
CdLS cell lines do not display sister chromatid cohesion abnormalities (Castronovo et al., 2009; Revenkova et al., 2009) indicating that the molecular mechanism of CdLS is not directly linked to the disruption of sister chromatid cohesion. Instead, considerable evidence suggests that CdLS etiopathology is due to the impaired ability of the cohesin to mediate other more dosage-sensitive cellular functions such as transcriptional regulation. NIPBL, HDAC8, and SMC1A mutant CdLS cell lines display transcriptional dysregulation; being conservative, the changes in gene expression are typically less than 1.5-fold (Cukrov et al., 2018; Deardorff et al., 2012; Liu et al., 2009; Mannini et al., 2015). Interestingly, this modest transcription dysregulation is in agreement with proteomic published data (Gimigliano et al., 2012). Furthermore, hundreds of genes are weakly transcriptionally dysregulated in the mouse, Drosophila and zebrafish CdLS model organisms (Kawauchi et al., 2009; Muto, Calof, Lander, & Schilling, 2011; Newkirk et al., 2017; Schaaf et al., 2009). These observations further support the notion that CdLS phenotype results from the collective action of many mild perturbations (Sarogni, Pallotta, & Musio, 2020). The molecular basis underlying gene dysregulation in CdLS is not clearly defined. It has been shown that mutant cohesin makes the binding chromatin/cohesin more stable and affects the recruitment of RNA polymerase II (Pol II) at both promoter and gene body of transcriptional active genes (Mannini et al., 2015; Revenkova et al., 2009).
The seven CdLS genes account for about 80% of cases, so the etiopathology of the remaining 20% of the patients with a clinical diagnosis of CdLS is unknown (Kline et al., 2018; Ramos et al., 2015). However, a correct diagnosis of CdLS is not readily made. Several observations indicate a high percentage of mosaicism in known CdLS genes that were previously excluded by Sanger sequencing using DNA obtained from peripheral blood samples (Ansari et al., 2014; Baquero-Montoya et al., 2014; Castronovo et al., 2010; Huisman, Redeker, Maas, Mannens, & Hennekam, 2013; Pozojevic et al., 2018). Furthermore, patients presenting CdLS or CdLS-like phenotype such as KBG (OMIM 148050), Wiedemann–Steiner (OMIM 605130), or Rubinstein–Taybi (RSTS2, OMIM 613684) have been described, notably, to harbor variants in chromatin-associated factors other than cohesin genes (Ansari et al., 2014; Aoi et al., 2019; Parenti et al., 2017; Woods et al., 2014; Yuan et al., 2015).
Here we report on the clinical and molecular characterization of two patients with features overlapping CdLS, for whom the analysis CdLS-causative genes did not identify any pathogenic variant. The subsequent use of exome sequencing allowed us to identify heterozygous variants in the ANKRD11 and EP300 genes. In addition, pathogenic variants led to low levels of both ANKRD11 and p300 proteins suggesting that this could contribute to the atypical form of CdLS. ANKRD11 and p300 are involved in gene expression regulation by chromatin remodeling (Chen et al., 2008; Gallagher et al., 2015). This report, presenting phenotypic overlap between CdLS, KBG, and RSTS, provides more evidence for the possibility that three distinct disorders share a common pathogenetic molecular mechanism.
2 MATERIALS AND METHODS
2.1 Subjects
All individuals in this study were examined by clinical geneticists and found to have clinical features consistent with CdLS. Clinical details were retrospectively reviewed based on recent clinical criteria (Kline et al., 2018; Table 1). Samples were collected after informed consent for research purposes, in accordance with the Declaration of Helsinki. Additional consent was obtained for the publication of photographic material identifying patients. Approval was granted by the Children Ethics Committee (CEP, protocol number 130/2016). All probands ascertained as CdLS were previously screened and were negative for pathogenic variants in genes responsible for CdLS.
| Patient A | Patient B | |
|---|---|---|
| Cardinal features (two points each if present) | ||
| Mutated gene | EP300 | ANKRD11 |
| Synophrys (HP:0000664) and/or thick eyebrows (HP:0000574) | 2 | 2 |
| Short nose (HP:0003196), concave nasal ridge (HP:0011120), and/or upturned nasal tip (HP:0000463) | — | — |
| Long (HP:0000343) and/or smooth philtrum (HP:0000319) | 2 | 2 |
| Thin upper lip vermilion (HP:0000219) and/or downturned corners of mouth (HP:0002714) | 2 | 2 |
| Hand oligodactyly (HP:0001180) and/or adactyly (HP:0009776) | — | — |
| Congenital diaphragmatic hernia (HP:0000776) | — | — |
| Suggestive features (one point each if present) | ||
| Global developmental delay (HP:0001263) and/or intellectual disability (HP:0001249) | 1 | 1 |
| Postnatal growth retardation (<2 sD) (HP:0008897) | — | — |
| Microcephaly (prenatally and/or postnatally) (HP:0000252) | 1 | 1 |
| Small hands (HP:0200055) and/or feet (HP:0001773) | — | 1 |
| Short fifth finger (HP:0009237) | — | — |
| Hirsutism (HP:0001007) | 1 | 1 |
| Clinical score | 9 | 10 |
- Note: ≥11 points, of which at least three are cardinal: classic CdLS; 9 or 10 points, of which at least two are cardinal: non-classic CdLS; 4–8 points, of which at least one is cardinal: molecular testing for CdLS indicated; ≤ 4 points: insufficient to indicate molecular testing CdLS; — denotes absence of feature.
- Abbreviations: CdLS, Cornelia de Lange syndrome.
2.2 Exome sequencing
DNA was extracted from peripheral blood lymphocytes by a standard nonorganic extraction procedure. SureSelect Human All Exon V7 kit (Agilent) was used for library preparation and exome enrichment, targeting about 60 Mb of human exonic content. Samples were quantified and quality-tested using the Qubit 2.0 Fluorometer (Invitrogen). Libraries were processed with Illumina cBot (Illumina) and sequenced on HiSeq2500 (Illumina), pair-end with 125 cycles per read. Reads were aligned to the UCSC Genome Browser hg19 reference sequence with the Burrows–Wheeler Aligner, and variant calling and genotyping were performed with the Genome Analysis Toolkit (Li & Durbin, 2009; McKenna et al., 2010). Variants were annotated by ANNOVAR (Wang, Li, & Hakonarson, 2010) and filtered with NCBI dbSNP v.151, the 1000 Genomes Project, NHLBI-ESP 6500 exome project, and AVSIFT (Liu, Jian, & Boerwinkle, 2011).
2.3 Antibodies
Commercially available antibodies used in this study are as follows: Actin (A300-491A, 1:5,000 dilution, Bethyl Laboratories), ANKRD11 (ab50852, against 50–200 region, 1:100 dilution, Abcam), p300 (A300-358A, against 950–1000 region, 1:10,000 dilution, Bethyl Laboratories). The ImageJ tool was used on scanned western blot images (Rueden et al., 2017; Schneider, Rasband, & Eliceiri, 2012).
2.4 Western blotting
Whole protein extracts were obtained from lymphoblastoid cell lines. Proteins were resuspended with lysis buffer, and protein concentration was estimated by the Bradford Protein Assay (Thermo Scientific). Proteins, 60 μg per lane, were separated by sodium dodecyl sulfate–polyacrylamide gel. The proteins were transferred to nitrocellulose membranes (Amersham) and incubated with the primary antibody. After removal of the unbound primary antibody, membranes were incubated with secondary antibody–peroxidase conjugate (Sigma), processed for detection by chemiluminescence (Amersham) and imaged on a Biomax film (Kodak). The AG09393 and AG14730 lymphoblastoid cell lines (purchased from Coriell Institute) were used as reference for Patients A and B, respectively.
2.5 Complementary DNA synthesis and quantitative real-time PCR
Complementary DNA was synthesized with SuperScript™ II reverse transcriptase using oligo dT (Invitrogen). Quantitative real-time PCR (RT-qPCR) analyses were performed using Rotor Gene 3000 (Corbett). Reactions were run in triplicate and normalized with respect to GAPDH. The AG09393 and AG14730 lymphoblastoid cell lines were used as reference. Since no difference was found in control cell lines, data were pooled. Primers used for mRNA expression analysis are listed in Table S1.
2.6 Statistical analysis
Data were analyzed by Student's t-test. p-values of <.05 were considered statistically significant.
3 RESULTS
3.1 Patient ascertainment
Patient A (Figure 1a) is the second child of an unrelated healthy couple. During the first months of life she showed feeding difficulties, with persistent crying, regurgitation, and poor weight gain. Gastroesophageal reflux symptoms manifested at night. She showed micrognathia, hirsutism, and synophrys. Her hands and feet were normal with the absence of broad thumb/hallux and large distal phalanges. In addition, she displayed profound hearing loss, both sensorineural and conductive. At the age of 6 years, neuropsychiatric evaluation showed moderate–severe cognitive impairment with an IQ of 49 (based on the Leiter-R scale) corresponding to a mental age of 36 months. Her growth measurements were small for her age. Her language was almost completely absent (producing about 20 words), and her personal autonomy was very limited. Finally, oppositional behaviors and low threshold of frustration tolerance were also present.
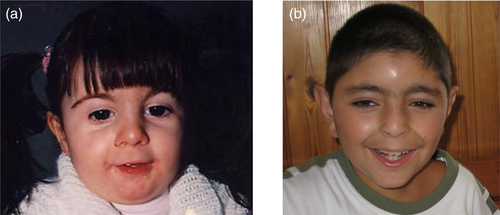
Patient B (Figure 1b) is the first child of an unrelated healthy couple. At birth, the proband had facial dysmorphism, long philtrum, thin lips, micrognathia, low anterior hairline, arched eyebrows, long eyelashes, synophrys, small hands, and hirsutism. At age 4 years, he was operated on for atrial septal defect. He also showed bilateral hypoacusis. His growth measurements were normal for his age. At the age of 7 years, neuropsychiatric evaluation showed moderate intellectual disability, specific behavioral issues such as aggression, defiance, extreme shyness, perseveration, and obsessive–compulsive behaviors. Both patients (A and B) were classified as non-classic CdLS (Table 1) when their clinical features were reevaluated based on clinical diagnostic criteria (Kline et al., 2018).
3.2 Exome sequencing
Since Sanger sequencing could not identify a disease-causing variant in CdLS-causative genes in any of the two patients, we used exome sequencing to sequence the patients' DNA.
Patient A was found to carry the c.4408_4409insA truncating frameshift variant in heterozygous status in exon 27 of the EP300 gene (RefSeq NM_001429.4) predicting a truncated protein sequence of 1,471 amino acids (aa), lacking part of the lysine (K) acetyltransferase (KAT) domain, instead of the full-length 2,414-aa p300 protein, p.(Met1470AsnfsTer3). This pathogenic variant was confirmed as de novo by Sanger sequencing (Figure S1a).
Patient B carried the pathogenic variant c.3224_3227del in heterozygous status in the ANKRD11 gene (RefSeq NM_013275.6) leading to p.(Glu1075GlyfsTer242) amino acid change. Neither parent had the variant indicating a de novo event (Figure S1b).
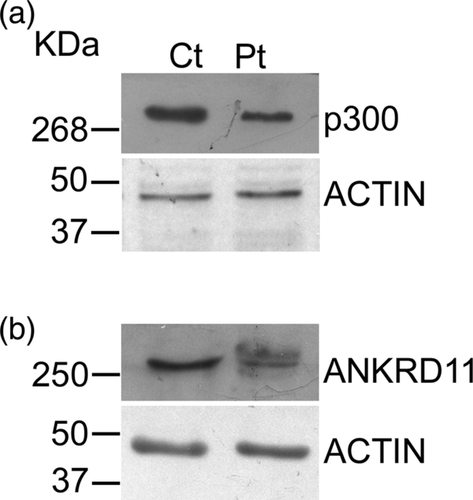
We next investigated the effect of the identified pathogenic variants on protein expression. Western blotting experiments showed that both c.4408_4409insA and c.3224_3227del pathogenic variants led to low levels of p300 and ANKRD11, respectively (Figure 2a,b, Figure S2a,b). RT-qPCR results revealed excellent agreement with western blotting experiments (Figure S3).
4 DISCUSSION
Exome sequencing has quickly become the method of choice for identifying non-high-frequency variations such as rare and de novo pathogenic variants in protein-coding exomes. In this study, exome sequencing was employed for the mutational screening of two probands with a clinical diagnosis of CdLS spectrum. By using this approach, we were able to identify the c.4408_4409insA and c.3224_3227del pathogenic variants in the EP300 and ANKRD11 genes that are associated with RSTS and KBG syndromes, respectively. To our knowledge, the variant in the EP300 has been described for the first time while that in the ANKRD11 gene has been recently reported in a patient with Coffin–Siris overlapping syndrome (Miyatake et al., 2017), adding more evidence that distinct disorders share a common genetic mechanism.
RSTS is a rare genetically heterogeneous multiple anomaly syndrome, and it is caused by pathogenic variants in the CREBBP (RSTS1) and EP300 (RSTS2) genes. According to HGMD (updated to October 2019), 21.3% (84 out of 394 variants) of clinically recognized RSTS cases carry EP300 pathogenic variants. EP300 variants have been associated with a milder phenotype, especially regarding the skeletal defects (Bartholdi et al., 2007; Bartsch et al., 2010; Fergelot et al., 2016; Foley, Bunyan, Stratton, Dillon, & Lynch, 2009; Tsai et al., 2011). KBG syndrome, a rare genetic disorder, is associated with the loss of function variants in the ANKRD11 gene, which encodes a chromatin regulatory protein (Gallagher et al., 2015). Our finding that ANKRD11 and EP300 inactivating pathogenic variants could be leading to low levels of the respective proteins suggests that this phenomenon results in an atypical form of CdLS. In this context, the EP300 and ANKRD11 pathogenic variants lead to premature termination codons, mRNA could be degraded by a nonsense-mediated decay mechanism to avoid the translation and accumulation of truncated proteins.
Although classic CdLS, KBG, and RSTS are unique conditions that are unlikely to be confused with one another, the differential diagnosis is more complicated in borderline-mild phenotypes (Avagliano et al., 2020; Kline et al., 2018). Until now, nine CdLS-like patients carrying variants in the EP300 or ANKRD11 genes have been described (Ansari et al., 2014; Aoi et al., 2019; Parenti et al., 2016; Woods et al., 2014), and clinical features were reported for six of them (Aoi et al., 2019; Parenti et al., 2016; Woods et al., 2014). Detailed findings of these CdLS-like patients, including our two, are shown in Tables S2 and S3. Most of the pathogenic variants are deletions, with the exception of two single nucleotide changes and a nucleotide insertion. Clinical reevaluation of our patients showed that some clinical signs, such as cognitive impairment, synophrys, microcephaly, and thin upper lip, and the absence of macrodontia or fused incisors suggested the clinical diagnosis of CdLS. This diagnosis was confirmed for the patient carrying the ANKRD11 variant, also in accordance with the recent CdLS consensus statement (Kline et al., 2018). Instead, the patient harboring EP300 variant was diagnosed as RSTS2. Since increasing data show that CdLS, KBG, and RSTS share these phenotypic features (Ansari et al., 2014; Aoi et al., 2019; Parenti et al., 2016; Woods et al., 2014), the testing for both EP300 and ANKRD11 variants in probands with features of CdLS should be warranted and included on panels for CdLS testing.
The finding that ANKRD11 and EP300 pathogenic variants cause a phenotype that mimics CdLS is intriguing. p300 functions as histone acetyltransferase that regulates transcription via chromatin remodeling and as a transcription co-factor by bridging transcription factors and intergenic RNA polymerase II (Chen et al., 2008; Roelfsema et al., 2005). Furthermore, it has been shown that p300, NIPBL, and the core cohesin proteins co-occupy enhancer regions in different cell types (Chen, Morris, & Mitchell, 2012; Kagey et al., 2010). Also ANKRD11 is a chromatin-associated/modifying protein that is particularly involved in the inhibition of the transcriptional activation of nuclear receptor targets genes via the recruitment of histone deacetylase proteins (Gallagher et al., 2015; Sirmaci et al., 2011; Zhang et al., 2004). Altogether, these observations indicate that perturbations in chromatin remodeling pathways, which include cohesin, and the EP300 and ANKRD11 genes, lead to human diseases and that their phenotypic effects might mimic one another. In this view, overlapping disorders can develop from a common genetic mechanism of disrupting the transcriptional machinery. This notion is supported by the observation that CdLS and CdLS-like cell lines display gene expression dysregulation (Deardorff et al., 2012; Izumi et al., 2015; Liu et al., 2009; Mannini et al., 2015; Olley et al., 2018). In summary, pathogenic variants in the EP300 and ANKRD11 can result in a CdLS-like phenotype overlapping that of other chromatin-dysregulated disorders.
ACKNOWLEDGMENTS
This work was supported by a grant from Fondazione Pisa (120/16) to A.M., grant from the Spanish Ministry of Health—Fondo de Investigación Sanitaria (FIS) [Ref. PI19/01860] and the Diputación General de Aragón (Grupo Reconocido B32_17R).
CONFLICT OF INTEREST
Authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
F.C., P.S., and A.P.: performed both exome and Sanger sequencing, and western blotting experiments; S.R., M.A., and C.M.: evaluated patients and provided clinical data; A.L., B.P., F.J.R., and J.P.: interpreted patient's data and contributed to the manuscript writing; A.M.: contributed to the concept, design, and manuscript writing. All authors commented on the draft and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data providing the evidence of the study is available from the corresponding author upon reasonable request.




