Extending the phenotypic spectrum of Bohring-Opitz syndrome: Mild case confirmed by functional studies
Funding information: Catalan Government, Grant/Award Number: 2014SGR932; CIBERER, Grant/Award Number: U720; Ministerio de Economia y Competitividad de Espana, Grant/Award Number: SAF2016-75948-R
Abstract
Bohring-Opitz syndrome (BOS) has been described as a clinically recognizable genetic syndrome since 1999. Clinical diagnostic criteria were established in 2011 and include microcephaly, trigonocephaly, distinctive craniofacial dysmorphic features, facial nevus flammeus, failure to thrive, and severe developmental delays. The same year, different de novo heterozygous nonsense mutations in the ASXL1 were found in affected individuals. Since then, several cases have been reported confirming the association between this chromatin remodeling gene and BOS. Most affected individuals die in early childhood because of unexplained bradycardia, obstructive apnea, or pulmonary infections. Those that survive usually cannot walk independently and are nonverbal. Some have had success using walkers and braces in late childhood. While few are able to speak, many have been able to express basic needs using communication devices as well as gestures with associated basic vocalizations. In this article, we present a mild case of BOS with a de novo pathogenic mutation c.1720-2A>G (p.I574VfsX22) in ASXL1 detected on whole-exome sequencing and confirmed by functional analysis of the messenger RNA splicing pattern on the patient's fibroblasts. She has typical dysmorphic features and is able to run and walk independently as well as to communicate with basic sign language.
1 INTRODUCTION
Bohring-Opitz syndrome (BOS), also known as Oberklaid-Danks syndrome or C-like syndrome (MIM605039), is a clinically recognizable genetic syndrome described for the first time in Bohring et al. (1999) Twelve years later, in 2011, diagnostic criteria were established by Hastings et al. including microcephaly, trigonocephaly, distinctive craniofacial dysmorphic features, facial nevus flammeus, failure to thrive, and severe developmental delays (Hastings et al., 2011). Craniofacial features include palatal abnormalities, prominent eyes, hypoplastic supraorbital ridges, upslanting palpebral fissures, depressed nasal bridge, anteverted nares, and low-set posteriorly angulated ears. The same year Hoischen et al. (2011) found the association between mutations in the ASXL1 gene and BOS after performing whole-exome sequencing (WES) in combination with direct sequencing and found different de novo heterozygous nonsense or frameshift mutations.
Congenital anomalies like corpus callosum defects and retinal and optic nerve abnormalities are frequently reported in BOS. Seizures are common as well as truncal hypotonia with hypertonia of the extremities. Affected patients assume a typical posture of the upper limbs including ulnar deviation of the wrists and/or fingers at the metacarpophalangeal joints. Russell et al. (2015) recommended Wilms tumor surveillance every 3 months until age 8 given the link between ASXL1 and myelodysplastic conditions. Severe feeding problems are common at the beginning of infancy, and most affected individuals die in early childhood because of unexplained bradycardia, obstructive apnea, or pulmonary infections. The ones who survive usually cannot walk independently and are nonverbal. Some have had success using walkers and braces in late childhood. While few are able to speak, many have been able to express basic needs using augmentative and alternative communication devices as well as gestures with associated basic vocalizations (Russell, Tan, & Graham Jr, 2018).
Additional sex comb-like1 (ASXL1) is known as a chromatin modulator that plays dual functions in transcriptional regulation depending on the cell type. Recent studies using Asxl1 knockout mice revealed its importance in proliferation and differentiation of hematopoietic progenitor cells, and in the development of organs (An et al., 2019). In this article, we present a mild case of BOS with a de novo pathogenic mutation c.1720-2A>G (p.I574VfsX22) in ASXL1 detected on WES and confirmed by the analysis of the messenger RNA splicing pattern on fibroblasts.
2 CASE REPORT
Our patient was born full-term via vaginal delivery to a G1, P0, 22-year-old mother and 29-year-old father of Ethiopian descent. Prenatal and family history were unremarkable. Birth parameters included weight 2.83 kg (13th percentile), length 45.7 cm (sixth percentile), and head circumference (HC) 34 cm (eighth percentile). Her newborn course was uncomplicated, and she passed her newborn hearing screen. She was noted to have glabellar nevus flammeus as an infant, which faded with age. She sat up at 7 months, crawled at 11 months, babbled at 12 months, and stood at 14 months. At 14 months, she was found to have a large cup to disc ratio on ophthalmological examination after exotropia was noted. She began to have seizures at 17 months including complex febrile and unprovoked seizures which typically occurred every few months. An electroencephalogram suggested a potential deep seizure focus from the left occipital/posterior quadrant region. Brain MRI demonstrated mild diffuse thinning of the corpus callosum, moderately small optic nerves and chiasm, mildly small pons, prominence of the left lateral ventricle likely reflecting mild left-sided periventricular white matter volume loss or hypogenesis, and no clear epileptogenic focus. An echocardiogram did not reveal cardiac disease.
She presented to our clinic at 19 months of age with global developmental delay. Her weight was in the 40th percentile, length in the 10th percentile, and HC in the 30th percentile. On physical examination, she was noted to have mild coarsening of the facial features, synophrys, upslanting palpebral fissures, prominent eyes, depressed nasal bridge, anteverted nares, narrow and high arched palate, widely spaced teeth, genu valgum, one hypopigmented macule on the chest, hirsutism, increased sandal gaps on both feet, and mild hypotonia (Figure 1; Figures S1–S4). She walked at 20 months of age and was able to climb the stairs with assistance at 24 months. At age 3, she was pointing and feeding herself with her hands, had balance issues, and had difficulty running. She was diagnosed with autism spectrum disorder at 4 years 3 months. At that time, she was able to respond to her name but had difficulty with eye contact. She had repetitive behaviors including spinning in circles, clapping her hands, and slamming doors.
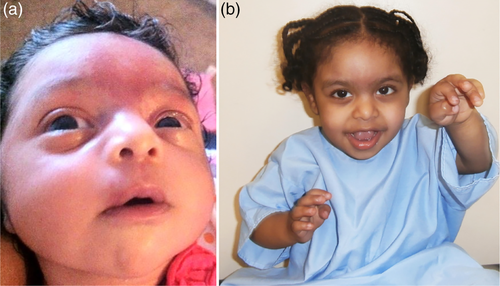
Growth parameters at the most recent physical examination at age 5 included weight in the 88th percentile, height in the 11th percentile, and HC in the 54th percentile. She is nonverbal but will point and sign for “more.” She is hyperactive and has frequent tantrums. She can wave and will pull a parent to what she wants. She needs assistance with dressing and brushing her teeth and is not toilet-trained. She will only scribble with a crayon and does not typically play with toys and will throw them instead. She can go up and down stairs with alternating feet and runs slowly. She is seizure-free on oxcarbazepine and levetiracetam, and her Wilms tumor surveillance has been negative so far. She attends special education preschool and receives speech, occupational, and physical therapies.
3 MATERIALS AND METHODS
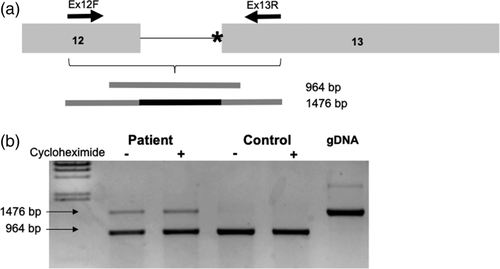
A CMA SNP array (CytoScan® Dx) was performed to detect copy-number variants. Gene panels were done by Massively Paralleled Sequencing using TruSight One kit (v1.0). WES was performed using the Illumina platform for next-generation sequencing. The exome capture is performed with NimbleGen reagents using a HGSC custom-designed capture reagent called VCRome 2.1. Patient and control's fibroblasts were obtained after signed consent and cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Life Technologies) and 1% streptomycin–penicillin (Gibco, Life Technologies, Carlsbad, California) and were maintained at 37°C and 5% of CO2. Cycloheximide (Sigma-Aldrich) treatment was applied in a concentration of 1 mg/ml in DMEM during 6 h. When confluence was reached, the RNA was extracted with the High Pure RNA Isolation Kit (Roche, Switzerland). RNA was then retrotranscribed using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, California). Amplification of the cDNA region containing the end of exon 12 and the beginning of exon 13 was performed by PCR using specific primers. The different isoforms obtained were cloned to a pGEM®T-easy vector (Promega, Madison, Wisconsin) following the manufacturer's instructions. The resulting plasmids were sequenced using the Sanger method by the CCitUB genomic services (Parc Científic, Barcelona). All protocols were approved by the Ethics Committee of the Universitat de Barcelona (IRB00003099), and all methods were performed in accordance with the relevant guidelines and regulations.
4 RESULTS
Initial genetic testing workup included a negative SNP chromosomal microarray, comprehensive epilepsy panel, and Noonan spectrum disorder panel. WES analysis showed three de novo variants: c.1720-2A>G in ASXL1 gene (ENST00000375687), c.253G>A in the STAG1 gene (ENST00000383202), and c.299_300del in the BACH1 gene (ENST00000399921) (Table S1). Several other variants of unknown significance were reported in autosomal dominant genes on testing, but all were inherited from an unaffected parent. RNA analysis from fibroblasts and Sanger sequencing of each band showed that the mutant allele led to the full retention of intron 12 (Figure 2). The aberrant transcript generated was not affected by the nonsense mediated decay (NMD) process, as no differences were observed when the culture was performed in the presence of cycloheximide.
5 DISCUSSION
Variants previously associated with BOS are truncating de novo mutations mainly located in exon 13 (Hoischen et al., 2011; Russell et al., 2018; Urreizti et al., 2016), like the c.1720-2A>G mutation found in our patient. Functional studies on fibroblasts from the patient validated the functional implication of this change. The variant caused a change in the reading frame that would lead to a premature stop codon after 21 residues (p.I574VfsX22). The fact that no exon–exon junction remains downstream of the premature stop codon is consistent with the lack of NMD. This de novo heterozygous intronic mutation in ASXL1 is affecting the canonical acceptor splice site at intron 12. The resulting protein is predicted to be truncated and will lack the C-terminus part of the protein. For all the reasons stated above, the mutation is considered to be pathogenic.
In many cases, WES will frequently identify several variants of unknown significance including more than one de novo variant, therefore muddling the ability to identify a unifying diagnosis. As with the present case, our patient's medical history alone did not meet strict clinical criteria for BOS and further studies were necessary to confirm her diagnosis. In fact, our patient has been previously reported as “patient 3” in Yuan et al. at the beginning of 2019 in a large cohort of patients with cohesinopathies, as a possible Cornelia De Lange like phenotype (Yuan et al., 2019). This manuscript indicates that the majority of disease-causing mutations described in STAG1 were loss of function changes and missense mutations mainly affecting specific domains. However, the mutation identified in our patient is far from these domains and predicted to be benign, neutral, or tolerated by PolyPhen 2, SNP and Go, PROVEAN, and SIFT. This prediction is most likely reflecting that valine and isoleucine are similar both in charge and size and suggesting a mild effect of the change on the protein structure and function. While the effect of this mutation of the patient's clinical presentation cannot be ruled out, we consider that this is, at most, a modifier mutation and not the main cause of the disease. Regarding BACH1, this gene acts as a transcription factor and plays a role in the regulation of oxidative stress pathway (Tan, Lim, Bennett, Shi, & Harper, 2013). So far, this gene has not been associated with any human disease and the knockout murine model was born at the expected Mendelian ratio and do not show any difference with the wild type (Ota, Brydun, Itoh-Nakadai, Sun, & Igarashi, 2014).
Although there has been at least one mild BOS case reported in the literature (Hoischen et al., 2011), to our knowledge, she is the first genetically confirmed patient with mild BOS who is able to communicate via sign language and walk independently whose mutation has been confirmed by functional studies. While she has typical facial dysmorphic features, she lacks the history of failure to thrive, BOS posture, severe intellectual impairment, microcephaly, and trigonocephaly. The early diagnosis of BOS is important to decrease the mortality rate over time (Russell et al., 2018). However, most reported patients have a severe phenotype including the BOS posture and severe developmental delays, which was not present in our patient. The application of WES in clinically unrecognizable genetic syndromes has not only significantly helped the early diagnosis of rare and new genetic syndromes but has also broadened the clinical spectrum of several known genetic conditions like in our case.
ACKNOWLEDGMENTS
The authors thank the patient and her family for their wholehearted collaboration. Funding was from Catalan Government (2014SGR932), Spanish Ministerio de Economía y Competitividad (SAF2016-75948-R) and from CIBERER (U720).
CONFLICT OF INTEREST
None.




