Novel and lethal case of cardiac involvement in DNM1L mitochondrial encephalopathy
Abstract
Pathogenic DNM1L mutations cause a mitochondrial disorder with a highly variable clinical phenotype characterized by developmental delay, hypotonia, seizures, microcephaly, poor feeding, ocular abnormalities, and dysarthria. We report the case of an 8-month-old female with autosomal dominant, de novo DNM1L c. 1228G>A (p. E410K) mutation and mitochondrial disorder, septo-optic dysplasia, hypotonia, developmental delay, elevated blood lactate, and severe mitochondrial cardiomyopathy leading to nonischemic congestive heart failure and cardiogenic shock resulting in death. This case suggests that cardiac involvement, previously undescribed, can be a clinically important feature of this syndrome and should be screened for at diagnosis.
1 CASE REPORT
A female infant was born at term to a 32-year-old G8P3 mother with gestational diabetes and otherwise unremarkable prenatal course. The patient's parents were healthy with no known family history of congenital metabolic, neurologic, or cardiac disease on full three-generation pedigree, and had previously had two healthy normally developing children together (a 12-year-old male and a 9-year-old female). At 4-months of age the infant was noted to be microcephalic and was diagnosed with failure to thrive; by 6-months of age gross motor delay (including truncal hypotonia), increased startle response, decreased visual tracking, and lactic acidosis (baseline 2.5–4.6 mmol/L) had developed. Brain magnetic resonance imaging (at 6 months of age) was interpreted as having reduced white matter volume with myelination normal for age, which along with her ophthalmologic evaluation supported a diagnosis of septo-optic dysplasia. Ammonia was within normal limits (41 μmol/L), and other biochemical studies were not performed (including plasma and urine organic acids).
The infant was evaluated by a clinical geneticist and subsequent trio whole exome sequencing revealed a de novo likely pathogenic variant of DNM1L leading to a diagnosis of autosomal dominant DNM1L-related mitochondrial encephalopathy. Sanger sequencing confirmed a missense mutation DNM1L c. 1228G>A (p. E410K) in coding exon 11 of the DNM1L gene. This alteration is not observed in population databases and the altered amino acid is highly conserved across all vertebrates reported. The p. E410K alteration is predicted to be probably damaging by Polyphen and deleterious by SIFT. Previous work has shown that p. E410 is located in the domain of DNM1L that is important for tetramerization of the protein (Chang et al., 2010) and the substitution of a lysine at position 410 would thus be predicted to produce disease via a dominant-negative mechanism (Von Spiczak et al., 2017). There were no other notable findings/alterations found on whole exome sequencing.
At 8 months of age she presented to a local emergency department with new onset poor feeding, diarrhea, and decreased urine output. Fluid resuscitation was administered for suspected dehydration. She subsequently decompensated with apparent cardiogenic shock and an urgent transthoracic echocardiogram revealed a dilated left ventricle (LV) with decreased systolic function (ejection fraction [LVEF] 35%, fractional shortening 16%) and chest radiograph demonstrated pulmonary edema. Milrinone, diuretics, and respiratory support were initiated, and she was transferred to a tertiary care center. On arrival her cardiac function remained tenuous with significant LV dysfunction on repeat echocardiogram (LVEF 32%) but clinical status improved and she was able to be weaned from respiratory support and was able to begin orally feeding again while being maintained on diuretics and milrinone. The following evening in setting of a blood transfusion, she developed respiratory distress and clinically worsening cardiac output. Despite the administration of additional diuretics, her respiratory distress and perfusion worsened. Bilevel positive pressure respiratory support was provided and low dose epinephrine was initiated for support of acutely worsening heart failure. At that time, the parents expressed a desire to avoid more invasive support with redirection of efforts to comfort care. Resuscitation was not escalated further and the patient expired in the mother's arms within several hours.
Given the temporal relationship of clinical worsening to the administration of blood products, standard postmortem laboratory investigation was undertaken and showed no evidence of hemolysis; culture of the residual blood product was negative. Autopsy showed features consistent with described DNM1L mitochondrial disorder with severe mitochondrial encephalopathy. Examination with light microscopy showed abundant neurons with enlarged mitochondria and reduced myelination of subcortical matter in the frontal lobe (Figure 1). Additionally, the skeletal muscle showed features of myopathy, characterized by abnormal mitochondria and muscle fiber atrophy. Evaluation of the heart revealed mitochondrial cardiomyopathy characterized by abundant cardiac myocytes with enlarged mitochondria, separation of myofibrils, and interstitial fibrosis (Figure 2). Additionally, there was subendocardial and endocardial fibrosis, ventricular hypertrophy, and ventricular dilation likely due to demand ischemia from congestive heart failure. Liver congestion from right heart failure and diffuse alveolar damage due to congestion from left-sided heart failure were also present.
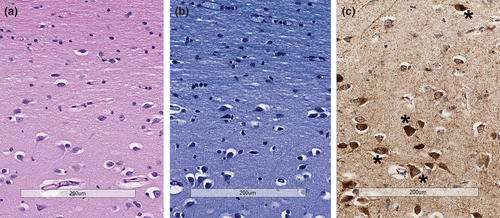
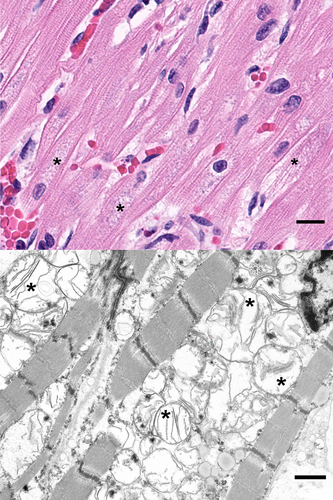
2 DISCUSSION
To our knowledge cardiac involvement has not previously been described in DNM1L mitochondrial disease, however, it appeared to play a critical role for this patient. Although the patient's demise was temporally associated with blood product administration, transfusion-associated cause of her deterioration was deemed unlikely. Radiographic findings where not consistent with transfusion-related acute lung injury (TRALI.) Acute hemolytic transfusion reaction does not appear likely given the lack of hemolysis on postmortem laboratory analysis. She also had no signs of angioedema or skin findings consistent with acute allergic reaction. The cause of her deterioration appears to have been due to acute exacerbation of nonischemic congestive heart failure with cardiogenic shock ultimately resulting in death. The presence of hypertrophic cardiomyocytes with swelling consistent with a mitochondrial abnormality point to her known DNM1L mutation as the etiology of the fatal cardiomyopathy.
The DNM1L gene encodes dynamin-1-like protein, which is a dyamin-related GTPase that plays an essential role in fission of mitochondria and peroxisomes (Kniffin, 2011; Sherman & Hamosh, 1999; U.S National Library of Medicine, 2019a; U.S National Library of Medicine, 2019b; UnitProtKB, 2005). This process allows mitochondria to replicate during cell division, allow for the transport and distribution of mitochondria, and the elimination of damaged mitochondria. Clinically DNM1L encephalopathy syndrome varies in severity (Fahrner et al., 2016), including developmental delay, hypotonia, microcephaly, poor feeding, ocular abnormalities (nystagmus, optic atrophy), seizures, and lactic acidosis. Treatment of DNM1L is supportive and individualized.
While there is no known cardiac involvement in humans with the DNM1L, one study described mutations in the DNM1L gene leading to autosomal dominant dilated cardiomyopathy (DCM) and ultimately congestive heart failure in “Python” mouse models (Ashrafian et al., 2010; El-Hattab, Suleiman, Almannai, & Scaglia, 2018). The study investigated single gene disorders leading to cardiac remodeling and DCM. Mice with the DNM1L mutation displayed reduced levels of mitochondrial complexes in cardiac myocytes implying cardiac ATP depletion. The authors suggest that mutation in the DNM1L gene leads to impaired mitochondrial remodeling in cardiac myocytes and ultimately DCM. However, the mice with this mutation did not develop (recognized) neurologic or skeletal manifestation of the DNM1L mutation, unlike the syndrome described clinically in humans. Previous reports suggest disruption of mitochondrial dynamics in progenitor cells can compromise differentiation of cardiac cells and ultimately lead to ineffective contractility. Cardiac differentiation of stem cells involves transformation of progenitor cells with relatively low energy requirements (able to be sustained by anaerobic metabolism) to cardiomyocytes with high energetic needs. Development and differentiation into sacromeres requires effective genetic reprogramming and involves genes with roles in mitochondrial fission. DNM1L is one such gene that encodes DRP1 protein. (Chung et al., 2007). Previously DNM1L mutations have been observed to predominantly effect the nervous system with speculation that embryonic Purkinje cells are highly dependent on mitochondrial division (Verrigni et al., 2019). Similarly, it is possible that disruption of mitochondrial oxidative metabolism in cardiac progenitor cells may impair sarcomerogensis and repair of cardiomyocytes, and ultimately results in ineffective contractility (Chung et al., 2007). In this patient's case the DNM1L c. 1228G>A (p. E410K) mutation could conceivably impair cardiac differentiation through impaired protein tetramerization and resultant insufficient energy supply and thereby result in ineffective cardiomyocytes and ultimately cardiomyopathy.
Knowledge of potential for cardiac involvement for patients with DNM1L is important for clinical management. This case implies evaluation and ongoing care of patients with DNM1L associated mitochondrial encephalopathy should involve cardiac screening and monitoring. Whether early intervention for and chronic management of the cardiomyopathy can improve outcomes in these patients, however, is unknown.
ACKNOWLEDGMENTS
There are no other contributors to acknowledge.
CONFLICT OF INTEREST
The listed authors have no conflict of interest (financial, relationship, or otherwise) to disclose.




