Comments on: A de novo in-frame deletion of CASK gene causes early onset infantile spasms and supratentorial cerebral malformation in a female patient
Bozarth, Foss, and Mefford (2018) recently published a case in the American Journal of Medical Genetics, Part A with an in-frame valine deletion in the Src homology 3 (SH3) domain of calcium/calmodulin-dependent serine protein kinase (CASK) (CASKVal727del) in a subject with infantile spasms, hypsarrhythmia on electroencephalogram, and a lack of both microcephaly and pontocerebellar hypoplasia per Magnetic resonance imaging (MRI). The presentation is highly atypical for a CASK variant identified as pathogenic, since prior to this, all individuals with confirmed heterozygous pathogenic mutations in CASK have cerebellar hypoplasia and/or microcephaly, but this particular subject exhibited neither. We recently reported a missense mutation (p.Leu209Pro) in CASK's N-terminal CaM kinase (CAMK) domain (CASKL209P) in a girl with bilateral retinal dystrophy and optic nerve atrophy (LaConte et al., 2019). This subject, also heterozygous for the CASK variant of interest, did display microcephaly and cerebellar hypoplasia, which prompted us to explore the possibility that the p.Leu209Pro mutation led to abolition of all CASK function. We found that CASKL209P tends to misfold and is no longer able to interact with its known binding partner, Mint1. Due to the distinctive phenotype ascribed to CASKVal727del by Bozarth et al. but not noted in the comprehensive clinical characterization available to us of the individual with the CASKL209P variant, we were interested to compare, at a biochemical and structural level, the behavior of CASKVal727del with CASKL209P.
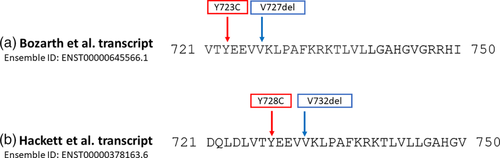
We generated a plasmid to express CASK with the in-frame valine deletion mutation, but in doing so, we discovered a discrepancy in the identification of the valine as described in Bozarth et al. (2018). When using the CASK isoform 1 sequence (Ensemble ID ENST00000645566.1), the ClinVar database (Variation ID 423552) denotes the deleted valine as Val727 (NP_003679.2:p.Val727del), and it is preceded by another valine (Figure 1a). However, Bozarth et al. noted the deleted valine to be adjacent in sequence to the site of a previously described pathological variant, Y728C (erroneously described as Y728H in Bozarth et al., 2018; Hackett et al., 2010; LaConte, Chavan, & Mukherjee, 2014), defined using a different transcript (Ensemble ID ENST00000378163.6) and listed in ClinVar under Variation ID 29937 (Figure 1b). When using the numbering based on the CASK isoform 1 sequence, as done in defining the CASKVal727del, this variation is appropriately identified as Y723C (NP_003679.2:p.Tyr723Cys). Thus, the in-frame deletion reported by Bozarth et al. is not adjacent to the Y723C variation.
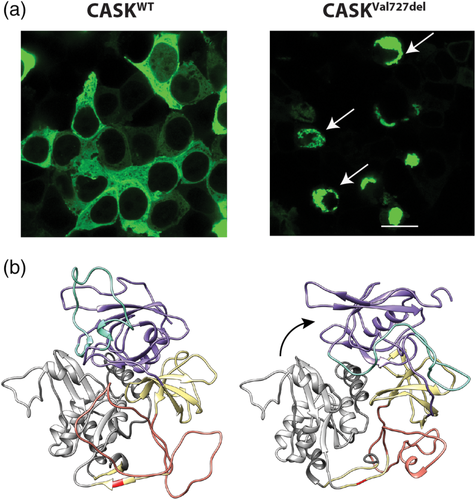
After successfully generating CASKVal727del and expressing in cell culture as described in LaConte et al., 2019, it is clear that, much like CASKL209P, CASKVal727del has a propensity to misfold (Figure 2a). In silico analysis of molecular dynamics (MD) simulations of CASK's PDZ-SH3-GK (PSG) supradomain containing the Val727del variation reveals no significant difference from the wild-type structure in overall radii of gyration sampled throughout the MD trajectories, suggesting that the in-frame deletion does not entirely destabilize the protein fold. The average structure representing the most populated conformation does, however, have an altered relationship between the PDZ and GK domains, offering a possible structural explanation for CASKVal727del's propensity to misfold (Figure 2b). Like CASKL209P, the soluble fraction of CASKVal727del maintains its critical interaction with neurexin (data not shown), and the interaction with Mint1 is unlikely to be impacted, since the variation is not in the domain known to interact with Mint1, suggesting that the interactions known to correlate with microcephaly are not disrupted in CASKVal727del, consistent with the Bozarth et al. findings. Our previous work with several missense mutations which produce misfolding (LaConte et al., 2014, 2018), in addition to the well-delineated phenotypic spectrum of pathogenic CASK variants over the last 10 years, suggests that the misfolding, in particular, of CASKVal727del is unlikely to be the source of the novel clinical phenotype described, since none of the other pathogenic misfolding mutations are associated with infantile spasms in the heterozygous condition. The propensity of CASKVal727del to misfold, however, may result in a partial loss of CASK function, whether simply due to reduced amount of protein or disrupted protein–protein interactions. Additionally, we have not observed any seizure-like phenotype in either the CASK(+/−) mice that we have generated or in other heterozygous mutation subjects we have encountered (DeLuca, Wallace, Trucks, & Mukherjee, 2016; Liang et al., 2017; Srivastava et al., 2016). Although it is still possible that CASKVal727del mutation may critically impact an as-of-yet untested but crucial interaction, based on our current observations, we believe that it is premature to directly associate the CASKVal727del with the phenotype described in Bozarth et al. The hypsarrhythmia in the subject noted by Bozarth et al. may instead result from synthetic phenotypic enhancement between a CASK variant likely to exhibit partial loss of function (CASKVal727del) and the concurrent mutation in the RAR Related Orphan Receptor A (RORA) gene, which is known to be associated with seizures. Therefore, rather than a distinct and novel clinical phenotype directly attributable to the CASKVal727del variant, this case report points to a possible dual diagnosis.
ACKNOWLEDGMENTS
This research was supported by from the NIH's National Eye Institute (Grant No. R01EY024712) to KM.
CONFLICT OF INTEREST
The authors declared that they have no conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




