Study of carrier frequency of Warsaw breakage syndrome in the Ashkenazi Jewish population and presentation of two cases
Abstract
Warsaw breakage syndrome (WABS), caused by bi-allelic variants in the DDX11 gene, is a rare cohesinopathy characterized by pre- and postnatal growth retardation, microcephaly, intellectual disability, facial dysmorphia, and sensorineural hearing loss due to cochlear hypoplasia. The DDX11 gene codes for an iron–sulfur DNA helicase in the Superfamily 2 helicases and plays an important role in genomic stability and maintenance. Fourteen individuals with WABS have been previously reported in the medical literature. Affected individuals have been of various ethnic backgrounds with different pathogenic variants. We report two unrelated individuals of Ashkenazi Jewish descent affected with WABS, who are homozygous for the c.1763-1G>C variant in the DDX11 gene. Their phenotype is consistent with previously reported individuals. RNA studies showed that this variant causes an alternative splice acceptor site leading to a frameshift in the open reading frame. Carrier screening of the c.1763-1G>C variant in the Jewish population revealed a high carrier frequency of 1 in 68 in the Ashkenazi Jewish population. Due to the high carrier frequency and the low number of affected individuals, we hypothesize a high rate of miscarriage of homozygous fetuses and/or subfertility for carrier couples. If the carrier frequency is reproducible in additional Ashkenazi Jewish populations, we suggest including DDX11 to Ashkenazi Jewish carrier screening panels.
1 INTRODUCTION
Warsaw breakage syndrome (WABS; MIM# 613398) is a rare recently described autosomal recessive cohesinopathy. The condition has been associated with severe pre- and postnatal growth retardation, microcephaly, intellectual disability, facial dysmorphia, and sensorineural hearing loss due to cochlear hypoplasia. Common dysmorphic features include epicanthal folds, cup-shaped ears, receding forehead, narrow bifrontal narrowing, and small face. Additional nonspecific dysmorphic features have been reported. Other features have been described including structural brain abnormalities, seizures, cardiac defects, renal abnormalities, recurrent infections, and hypopigmented and hyperpigmented patches (Van der Lelij et al., 2010; Capo-Chichi et al., 2013; Bailey, Fryer, & Greenslade, 2015; Eppley, Hopkin, Mendelsohn, & Slavotinek, 2017; Alkhunaizi et al., 2018; Bottega et al., 2019). WABS is caused by bi-allelic variants in the DDX11 gene. The product of the DDX11 gene is a member of the Superfamily 2 (SF2) helicases and contains an iron-sulfur (Fe-S) motif and a DEAD/DEAH box. Fe-S helicases have been shown to be vital for genomic stability and maintenance. Other Fe-S helicases, such as XPD, FANCJ, and RTEL1, have also been associated with human disease (Brosh Jr, 2013; Pisani, Napolitano, Napolitano, & Onesti, 2018).
DDX11 plays an important role in genomic stability and maintenance, as it is involved with sister chromatid cohesion, DNA repair, and normal chromatin structure (Pisani et al., 2018; Sun et al., 2015). Breakage studies of affected individuals show increased chromosomal breakage in the presence of mitomycin C (MMC). The breaks showed a “railroad” appearance from centromeric heterochromatin repulsion, and premature chromatid separation. These features have emerged as a unique cytogenetic profile consisting of a combination of features seen with Fanconi anemia and Roberts syndrome. This cytogenetic profile though is not consistent among all affected individuals with WABS (Alkhunaizi et al., 2018; Bailey et al., 2015; Capo-Chichi et al., 2013; Eppley et al., 2017; van der Lelij et al., 2010).
Heterozygous carriers or suspected heterozygous carriers have previously been reported to have various early onset cancers (Alkhunaizi et al., 2018; Van der Lelij et al., 2010). It is unknown whether there is an association with increased cancer risk and DDX11 carrier status, or if these cancers are present in these families by chance. Since association of increased cancer risk in carriers is not established, there are currently no guidelines for cancer surveillance and carrier status is not used as an indicator of possible cancer risk. Increased risk of cancer has been seen with carrier status of other breakage syndromes (Bogdanova et al., 2008; Seemanová et al., 2007).
To date, only 14 patients with WABS have been reported in the medical literature. All of these individuals have been of various ethnic backgrounds and most have different pathogenic variants. Here, we report two unrelated patients of Ashkenazi Jewish descent who are affected with WABS who are both homozygous for the same variant. Carrier screening in the Ashkenazi Jewish community revealed a high carrier frequency for this variant.
2 MATERIALS AND METHODS
2.1 Patients
Both patients were previously evaluated through clinical genetic services when whole exome sequencing was performed. Patients underwent genetic counseling and consent was obtained for testing. Patient 1 had whole exome sequencing performed at GeneDx, Gaithersburg, MD. Patient 2 had whole exome sequencing performed at Children's Hospital of Philadelphia, Philadelphia, PA. The patients were retrospectively noted to have similar features and homozygous variant in the DDX11 gene. The clinicians of the patients were connected through the Dor Yeshorim screening program. Written consent was obtained from both patients for publication of their clinical phenotype and genotype.
2.2 Carrier screening
We genotyped 26,015 individuals from different Jewish populations to determine the carrier frequency for the c.1763-1G>C variant in the DDX11 gene. Anonymous blood samples were obtained from the Dor Yeshorim screening program from December 2016 to December 2018 (Ekstein & Katzenstein, 2001). Samples were obtained at Dor Yeshorim screening locations around the world. Locations include the United States of America (New York, New Jersey, Maryland, California, Illinois, Florida, Ohio, and Michigan), Canada, Mexico, Argentina, Brazil, UK, Belgium, France, Switzerland, Austria, Australia, South Africa, and Israel. Individuals who typically use the Dor Yeshorim screening program represent Jews of all levels of Orthodoxy (Ekstein & Katzenstein, 2001). All participants provided written consent to be used for research purposes. The consent form included that patient material would be used for clinical testing and that excess material would be de-identified for use of research purposes to characterize single gene disorders in the Ashkenazi Jewish population. Genomic DNA was extracted and screened in accordance with a previously described high throughput TaqMan allelic discrimination genotyping method on the Fluidigm platform (Fedick et al., 2013). The percentage of derived carrier frequencies are considered to be more precise than the ratios, which were rounded to the nearest whole integral.
In the original data set, each sample was classified by self-identification as Ashkenazi Jewish, Sephardi Jewish, Ashkenazi Jewish/Sephardi Jewish, convert, or unknown. Samples from converts and unknown origin made up a total of 309 and were excluded from analysis.
To elucidate if c.1763-1G>C variant in the DDX11 gene was present at higher rates in Jews residing from various countries, we classified data based on self-reported ancestry of four grandparents. Individuals with only one, two, three, or all four grandparents from the same country were designated according to country of origin. Individuals who stated two or more countries were considered to be of mixed origin and were therefore removed from analysis. In cases where South Africa was provided as country of origin, the individuals were redefined as Lithuanian since South African Jews are primarily of Lithuanian origin (Meiner et al., 1991). Individuals who had Israel or United States stated in their ancestry were also removed from analysis, since the Jews residing in these countries often have mixed Ashkenazi Jewish origins. Finally, individuals who did not provide any information of grandparental origin or stated unknown were removed from analysis. These strict criteria of retaining samples with only one country of ancestral origin, which totaled 3,356 individuals, reduced the initial data set by 87%.
2.3 RNA studies
RNA sequencing was performed on Patient 2. RNA from whole blood was extracted by PAXgene Blood RNA Kit (Qiagen). Cytoplasmic and mitochondrial ribosomal RNA was depleted with Ribo-Zero Gold (Illumina). Globin Zero Kit (Illumina) was used to remove hemoglobin mRNA. Libraries were prepared using NEBNext Ultra RNA Library Preparation Kit for Illumina (New England Biolabs) and sequencing was performed on Illumina HiSeq 2×150 bp with single index. For case and control samples, 243,922,311 and 169,686,475 reads were generated, respectively. Mean quality score was 38.50 and 38.45 with 92.78 and 92.59% of bases having quality score above 30. Trimmomatic v.0.36 was used to trim adapter sequences and low-quality bases. Trimmed reads were mapped to GRCh38 reference genome using STAR aligner v.2.5.2b.
3 RESULTS
3.1 Clinical phenotype description
Patient 1 was a male born to nonconsanguineous parents of Ashkenazi Jewish descent. His father was of Polish origin and his mother was of Polish, Romanian, and Lithuanian origins. The family history is significant for renal cell carcinoma in the patient's father that was diagnosed at 44 years of age. The remainder of the family history was unremarkable. Pregnancy was complicated by placental abruption and the patient was born at 28 weeks gestation to a primigravida woman. The patient weighed 793 g at birth. He was kept in the neonatal intensive care unit for about 3 months to monitor for prematurity and had one episode where he stopped breathing and was revived. At the age of one, the patient was found to have severe bilateral sensorineural hearing loss and received right sided cochlear implant at 2 years of age. The patient developed a seizure disorder at 10 years of age. The seizures began as nocturnal and then started to occur during the day. The seizures were controlled with medications. At most recent evaluation at 21 years of age, he was managed on Keppra and Depakote. No further information about seizure classification was available. Brain MRI was performed when the patient was a child. The parents reported that the brain MRI was abnormal; however, reports were not available for our review. The patient had congenital hypothyroidism. He was managed on Synthroid. His thyroid antibodies were negative and his history was consistent with a mild dyshormonogenesis. At 20 years of age, the patient was diagnosed with Type 1 diabetes mellitus with hyperglycemia. He was managed on insulin. Patient also had a history of kidney stones.
The patient had developmental delays throughout his life. He started to walk at about 2.5 years of age and first words came at about 3–3.5 years of age. His speech developed slowly over the years. At 21 years of age, he could communicate his needs; however, his speech was not clear and would only speak in a new setting when prompted. He was diagnosed with speech apraxia at 6 years of age. He also had a diagnosis of autism spectrum disorder. The patient was educated in special programs and occupational, physical, and speech therapies. After he finished school, the patient began attending a day rehabilitation program.
The patient was first evaluated by clinical genetics when he was about 4 years old. Differential diagnosis at the time included Johanson-Blizzard syndrome and dysautonomia. The patient returned to genetics at 21 years of age. Clinical evaluation was significant for microcephaly (52 cm; <2nd %), short stature (156 cm; <3rd %), bilateral short mid phalanx of the 5th finger, bilateral clindodactyly, right single palmar crease, bilateral limitation of extension of the elbows, narrow palate, crowded teeth, and hypotelerism. No other dysmorphic features were noted.
Previous genetic testing included chromosome and microarray analysis that were reported as normal. Trio based whole exome sequencing performed at GeneDx identified the patient to be homozygous for the c.1763-1G>C pathogenic variant in the DDX11 gene (NM_030653.3). Each parent was identified to be a carrier for this variant. The patient was also found to be heterozygous for two pathogenic variants in cis in the POLG gene. The patient is thought to only be a carrier for POLG-related disorders, as his mother carries the same variants. These two variants are commonly found in cis and seen in affected individuals when the variants are in trans with a third pathogenic variant.
Patient 2 was a female born to nonconsanguineous parents of Ashkenazi Jewish descent. Her father was of Hungarian origin and her mother was of Polish origin. She was the third of seven children in her family. Family history was significant for four first trimester miscarriages of unknown etiology. The remainder of the family history was unremarkable. Pregnancy was complicated by intrauterine growth restriction and the patient was born at 32 weeks gestation via caesarian section. The patient weighed 624 g and was 33 cm long at birth. Head circumference was 22 cm at birth. Postnatal evaluation identified a sacral dimple, bilateral clinodactyly of the fourth toe, and a soft systolic murmur over the upper left sternal border but was otherwise unremarkable. The heart murmur resolved by 1 year of age; however, the patient never had a full cardiac work up. At 6 months of age, she was diagnosed with hypothyroidism, for which she was treated with Synthroid. At 3 months of age, she had bilateral inguinal hernia repair. Microcephaly first became a concern at 5 months of age. During infancy, the patient suffered from significant feeding problems and at 10 months she required a gastrostomy tube placement until it was removed at 10 years of age. She was then placed on a high caloric diet for 5 years. At 11 months, brain MRI showed bilateral cochlear hypoplasia, as well as simplified gyral pattern with microcephaly. Mild hypoplasia of the cerebellar vermis was also noted. At 6 months of age, Patient 2 was found to have bilateral profound sensorineural hearing loss and she received bilateral cochlear implant at the age of two. At 18 months, she was diagnosed with mild left exotropia, which did not require any treatment. She also suffered from constipation and would not achieve a bowel movement without MiraLAX and Benefiber. Patient had normal genitalia and pubertal development and achieved menarche at about 12 years of age. Patient 2 was noted to have congenital dislocation of her right elbow. Skeletal survey was normal. At 9 years of age, Patient 2 developed complex partial seizures.
The patient always had delayed development from early infancy, for which she received physical therapy, speech therapy, and occupational therapy. She started to walk at 2 years of age with an unstable gait and tiptoe walking. She spoke her first words at 24 months. She was toilet trained at the age of 6–7 years. At most recent clinical evaluation, the patient was 16 years old. She was known to have mild intellectual disability and attended a special education school. She continued to receive therapies with slow improvement. She still tiptoe walked, she could run, but she could not ride a bike or jump rope. Her speech had also improved. She could combine words and communicate her needs to the extent of 80%, but her speech was still unclear. She could write her own name, dress herself, and manage her own hygiene needs with little aid. The patient's parents believed that she did not have a concept of time. They also believed that she was anosmic, but they were unable to prove it.
Patient 2 had been followed by clinical genetics since the age of 1. Clinical evaluations have been significant for microcephaly (41 cm at 10 years of age; <2nd %), short stature (137 cm at 16 years of age; <3rd%), failure to thrive, stellate irises, arched eyebrows, synophrys, prominent nasal bridge with columella extending below the nares, long philtrum, and slightly high arched palate. When she was younger, the differential diagnosis included Seckel syndrome, other forms of dwarfism, and Johanson–Blizzard syndrome. She had a normal chromosome analysis and negative work-up for Bloom syndrome. Throughout her early childhood years, she developed several irregularly shaped café-au-lait macules mostly on her legs. This led to the suspicion of Fanconi anemia and neurofibromatosis Type 1. Neurofibromatosis Type 1 testing was negative and she had no axillary or inguinal freckling. Further testing for Fanconi anemia confirmed bone marrow failure, which included hypocellular marrow with trilineage hematopoiesis. Additionally, chromosome breakage studies performed at Cincinnati Children's Hospital did not identify any cells with breakage under DEB clastogen exposure. MMC clastogen exposure showed 26 out of 50 cells with breakage. Three of them had more than 10 breaks, which is within the range for patients with Fanconi anemia. However, genetic testing for a panel of 16 genes responsible for Fanconi anemia performed at Children's Hospital of Philadelphia was negative. Subsequently, whole exome sequencing analysis performed at Children's Hospital of Philadelphia revealed a splice site homozygous variant c.1763-1G>C in the DDX11 gene. Segregation analysis confirmed a recessive mode of inheritance and each parent was found to be a carrier.
3.2 Carrier screening
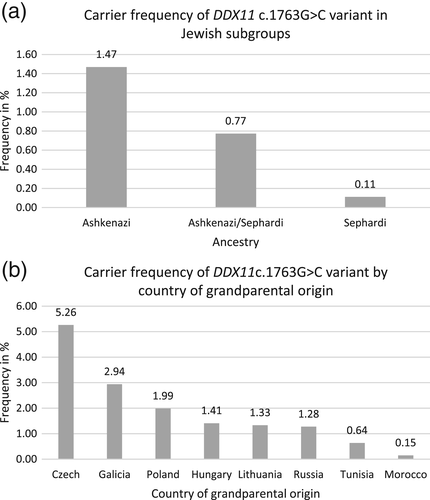
The observed carrier frequency of the c.1763-1G>C variant in the DDX11 gene in Jewish individuals reached 1.47% or 1 in 68 in Ashkenazi Jewish individuals, 0.77% or 1 in 129 in mixed Ashkenazi Jewish/Sephardi Jewish individuals, and 0.11% or 1 in 895 in Sephardi Jewish individuals (Figure 1a). Data classified by country of origin show the following carrier frequencies: Czechia (present day Czech Republic) 5.26% or 1 in 19, Galicia (parts of present day Poland and Ukraine) 2.94% or 1 in 34, Poland 1.99% or 1 in 50, Hungary 1.41% or 1 in 71, Lithuania 1.33% or 1 in 75, Russia 1.28% or 1 in 78, Tunisia 0.64% or 1 in 157, and Morocco 0.15% or 1 in 664 (Figure 1b). These carrier frequencies correspond to the following number of samples from each country: Czechia 1 carrier and 18 noncarriers, Galicia 1 carrier and 33 noncarriers, Poland 15 carriers and 740 noncarriers, Hungary 20 carriers and 1,397 noncarriers, Lithuania 1 carrier and 74 noncarriers, Russia 3 carriers and 232 noncarriers, Tunisia 1 carrier and 156 noncarriers, and Morocco 1 carrier and 663 noncarriers.
3.3 RNA sequencing
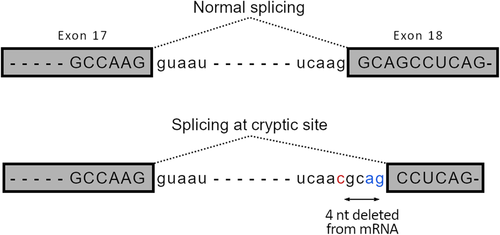
RNAseq data confirmed the c.1763-1G>C variant at the canonical splice-acceptor site in intron 17 of DDX11 (NM_030653.4; Figure 2). Visualization of mapped reads showed the utilization of a cryptic splice-acceptor in exon 18, leading to skipping of the first 4 nucleotides of normal exon 18 and resulting in a frameshift, which is predicted to cause abnormal protein product (Figure SS1).
4 DISCUSSION
We report the first two unrelated individuals of Ashkenazi Jewish descent known to be affected with WABS. Individuals who are homozygous for the c.1763-1G>C variant in the DDX11 gene appear to share intellectual disability, microcephaly, growth retardation, and hearing loss, with the previously described individuals with WABS (Table 1; Alkhunaizi et al., 2018; Bailey et al., 2015; Bottega et al., 2019; Capo-Chichi et al., 2013; Eppley et al., 2017; Van der Lelij et al., 2010). The importance of the DDX11 gene for genomic stability and maintenance has been described, with its involvement in sister chromatin cohesion, DNA repair, and normal chromatin structure (Pisani et al., 2018; Sun et al., 2015). Chromosomal breakage studies of affected individuals have shown increased breakage when exposed to MMC, as seen here in Patient 2.
| Patient 1 | Patient 2 | van der Lelij et al. (2010) (N = 1) | Capo-Chichi et al. (2013)(N = 3 siblings) | Bailey et al. (2015) (N = 1) | Eppley et al. (2017) (N = 2 siblings) | Alkhunaizi et al. (2018) (N = 5) | Bottega et al. (2019) (N = 2 siblings) | Total (N = 14) | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | M | F | M | M = 1 F = 2 |
F | F = 2 | M = 4 F = 1 |
F = 2 | M = 7 F = 9 |
| Ethnicity | Ashkenazi (Polish/Romanian/Lithuanian) | Ashkenazi (Hungarian/Polish) | Polish | Lebanese | British | Mixed European/Native American | Croatian/Italian Pakistani Saudi (n = 2) Egyptian |
NA | |
| Consanguinity | − | − | − | + | − | − | 4/5 | − | 7/16 |
| Developmental delay/intellectual disability | + | + | + | + | + | 2/2 | 5/5 | 1/2 | 15/16 |
| Hypotonia | NA | NA | NA | 2/2 | NA | NA | 1/5 | NA | 3/7 |
| Microcephaly | + | + | + | 3/3 | + | 2/2 | 5/5 | 2/2 | 16/16 |
| Intrauterine growth restriction | NA | + | + | 2/2 | + | 2/2 | 5/5 | 2/2 | 14/14 |
| Postnatal growth retardation/short stature | + | + | + | 3/3 | + | 2/2 | 5/5 | 2/2 | 16/16 |
| Sensorineural hearing loss | + | + | + | 3/3 | + | 2/2 | 5/5 | 2/2 | 16/16 |
| Cochlear hypoplasia | NA | + | + | 3/3 | + | 2/2 | 4/4 | 2/2 | 15/15 |
| Structural brain abnormalities | NA | + | NA | NA | NA | NA | 4/4 | 0/2 | 4/6 |
| Seizures | + | + | − | 0/3 | − | 0/2 | 1/5 | 0/2 | 3/16 |
| Congenital hypothyroidism | + | + | − | 0/3 | + | 0/2 | 0/5 | 0/2 | 3/6 |
| Diabetes mellitus | + | − | − | 0/3 | − | 0/2 | 0/5 | 0/2 | 1/16 |
| Feeding problems | − | + | − | 0/3 | + | 0/2 | 0/5 | 0/2 | 2/16 |
| Multicystic kidney | − | NA | NA | 0/2 | + | NA | 0/3 | NA | 1/7 |
| Congenital heart defects | − | NA | + | 1/3 | + | 0/2 | 2/5 | NA | 5/13 |
| Genitalia | − | − | − | NA | − | 0/2 | 2/5 | NA | 2/11 |
| Early puberty | − | − | − | NA | − | 2/2 | 0/5 | NA | 2/11 |
| Recurrent infections | − | − | − | NA | − | 2/2 | 2/5 | NA | 4/11 |
| Hypopigmented/ hyperpigmented patches | − | + | + | 0/3 | + | 2/2 | 2/5 | 2/2 | 6/16 |
| Facial dysmorphia | + | + | + | 3/3 | + | 2/2 | 5/5 | 2/2 | 16/16 |
| Skeletal abnormalities of fingers/toes | + | + | + | 3/3 | + | 2/2 | 5/5 | 1/2 | 15/16 |
| Other skeletal abnormalities | − | + | NA | NA | − | 2/2 | 2/5 | NA | 5/10 |
| Family history of early onset cancer | + | − | NA | NA | NA | NA | 2/4 | NA | 3/6 |
| Family history of multiple miscarriages | − | + | NA | − | NA | NA | 1/5 | NA | 2/10 |
- Abbreviations: F, female; M, male; N, number; NA, not available.
Both of our patients had a history of congenital hypothyroidism and had a seizure disorder. Both of these conditions have respectively only been described in one patient previously (Alkhunaizi et al., 2018; Bailey et al., 2015). We suggest expanding the phenotypic spectrum to include seizure disorder and congenital hypothyroidism.
The RNA studies performed in Patient 2 showed that the c.1763-1G>C splice site variant causes an alternative splice acceptor leading to a frameshift of the open reading frame, which is predicted to cause an abnormal protein product. The variant occurs immediately upstream of a functional domain, the DNA helicase (DNA repair), Rad3 type domain. This variant has been reported with an allele frequency of 86/10370 (0.829%) in individuals of Ashkenazi Jewish descent and an allele frequency of 104/282672 (0.037%) in total in the gnomAD browser. There are no reported homozygous individuals in population databases (Lek et al., 2016).
Our carrier screening analysis shows that this variant is common in the Ashkenazi Jewish population with a carrier frequency of 1 in 68 or 1.47%. We suspect that our carrier screening yielded a higher carrier frequency than gnomAD because the Jewish population screened was a religious Jewish community where individuals have a higher tendency to stay and marry within the community. The population that was screened though, represents a diverse population of Orthodox Jews from all over the world. Observed carrier frequencies for Chechia, Galicia, Lithuania, Tunisia, and Morocco should not be considered as absolute due to limited number of samples, whereas frequencies for Poland, Hungary, and Russia can be interpreted with confidence. The observed elevated carrier frequency in Jews of Polish origin is not statistically significant when tested by one-sided Fishers's exact test against frequencies from Hungary or Russia. Given the high carrier frequency identified in the Ashkenazi Jewish population, we have preliminary evidence that this is may be an Ashkenazi Jewish founder mutation. We therefore suspect that the five carriers of Sephardi Jewish descent to have unknown Ashkenazi Jewish ancestry in their lineage.
There are many founder mutations that have been identified for various disorders in the Ashkenazi Jewish population, for which carrier screening is widely performed. Carrier screening in the Ashkenazi Jewish community became wide spread in 1970's for screening for Tay-Sachs disease (Klugman & Gross, 2010). Since then, the American College of Medical Genetics and Genomics (ACMG) and the American College of Obstetrics and Gynecology (ACOG) have recommended several conditions to be screened or to be considered for screening, ideally prior to pregnancy, in individuals of Ashkenazi Jewish descent. In 2008, ACMG recommended carrier screening for familial dysautonomia, Tay-Sachs disease, Canavan disease, Fanconi anemia group C, Neimann-Pick type A, Bloom syndrome, mucolipidosis IV, and Gaucher disease Type 1 (Gross, Pletcher, & Monaghan, 2008). In 2009, ACOG recommended carrier screening for Canavan disease, cystic fibrosis, familial dysautonomia, and Tay-Sachs disease. The American College of Obstetrics and Gynecology listed additional conditions that they recommend to be considered for carrier screening (Rink, Romero, Biggio Jr, Saller Jr, & Giardine, 2017). All of these conditions have founder mutations in the Ashkenazi Jewish population and the carrier frequency in the Ashkenazi Jewish population of these conditions ranges from 1 in 18 to 1 in 168 (Gross et al., 2008; Rink et al., 2017). Many laboratories now offer expanded carrier screening panels, which include conditions that have much lower carrier frequencies in the Ashkenazi Jewish population (Mastantuoni et al., 2018; Shi et al., 2017). Uptake of carrier screening in the Ashkenazi Jewish population was positive and affected individuals for some of these conditions are rarely seen in the Ashkenazi Jewish community today (Klugman & Gross, 2010).
The carrier frequency that we observed of the c.1763-1G>C variant in the DDX11 gene is in the range of carrier frequencies of conditions recommended to be screened by both ACMG and ACOG. The carrier frequency of this variant observed in gnomAD also falls within the carrier frequency range of the conditions recommended to be screened. The American College and of Medical Genetics and Genomics recommends that additional conditions should be considered for addition to the recommended Ashkenazi Jewish carrier screening panel if the natural history of the disorder is understood, variants in the homozygous or compound heterozygous state have a significant risk of morbidity and/or mortality, and if the allele frequency in the Ashkenazi Jewish population is >1%. We show that individuals who are homozygous for the c.1763-1G>C variant in the DDX11 gene have a phenotype consistent with the described WABS and the allele frequency is >1% in the Ashkenazi Jewish population. Given that our data is derived from a religious community who self-reported their ancestry, additional carrier frequency studies should be performed in more diverse Jewish populations. If further carrier screening studies confirm that the carrier frequency of this variant is high in the Ashkenazi Jewish population, we suggest DDX11 to be considered for inclusion on Ashkenazi Jewish carrier screening panels.
Given the high observed carrier frequency and limited number of identified affected individuals, we hypothesize that there is a high rate of spontaneous abortion associated with homozygosity of the c.1763-1G>C variant and/or subfertility among carrier couples. There have previously been families reported with a history of multiple miscarriages, and here we report another family with a history of four spontaneous abortions (Alkhunaizi et al., 2018; van der Lelij et al., 2010). Using our observed carrier frequency, the expected birth rate of Ashkenazi Jewish individuals affected with WABS per 100,000 Ashkenazi Jewish individuals based on Hardy Weinberg is 5.40 live births. To date, there are only two individuals of Ashkenazi Jewish descent known to be affected with WABS. Absence of the DDX11 gene has been shown to be embryonic lethal in mice (Inoue et al., 2007; Sun et al., 2015). Mice embryos with knockout DDX11 were smaller, malformed, and had sparse cellularity when compared to wild type and heterozygote mice. DDX11 knockout mice embryos were also unable to form a proper placenta (Inoue et al., 2007). Previous hypotheses suggest that complete loss of DDX11 is not compatible with life and patients must carry at least one hypomorphic variant to be viable (Bottega et al., 2019). In the case of multiple miscarriages with variants that are hypomorphic, it is possible that additional unknown factors may influence the expression of DDX11 to establish if the fetus will be viable. Additional studies should be conducted to investigate the relationship between homozygous DDX11 variants and embryonic lethality. If further research demonstrates increased rate of miscarriage among homozygous DDX11 fetuses, we suggest that DDX11 carrier status to be included in the differential diagnosis of Ashkenazi Jewish couples with multiple spontaneous abortions of unknown etiology. The two live births described in this report, our patients, may represent the mild end of the spectrum of the DDX11 homozygous state. This phenomenon is similarly seen in other genetic conditions, such as Turner syndrome, where the majority of affected fetuses do not make it to term (Cockwell, MacKenzie, Youings, & Jacobs, 1991; Saenger, 1996). It is also possible that WABS is underdiagnosed in the Ashkenazi Jewish population and other populations. Greater uptake and access to genetic testing in these populations may identify additional individuals with WABS. The differential diagnosis for affected individuals may be broad, and genetic testing may be necessary to establish the correct diagnosis.
We describe another heterozygous individual with early onset cancer. The father of Patient 1 was diagnosed with renal cell carcinoma at age 44. There have been three previous families where heterozygous carriers or suspected heterozygous carriers have been diagnosed with various cancers (Alkhunaizi et al., 2018; van der Lelij et al., 2010). Further research should be conducted to explore the link of heterozygous carriers of DDX11 and an increased risk of cancer, especially due to the high carrier frequency in the Ashkenazi Jewish population. More detailed family histories of affected individuals can be obtained to identify additional carriers who may have early onset cancers. If carriers are at an increased risk of cancer, their medical management should be geared appropriately. Heterozygous carriers of other breakage syndromes, such as Nijmegen breakage syndrome, have been shown to have increased risk of cancer (Seemanová et al., 2007; Bogdanova et al., 2008). There are currently no National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for surveillance of DDX11 carriers. In the absence of surveillance guidelines and studies that confirm association of carrier status of DDX11 and cancer, we advocate no disclosure of the possible risk for cancer to carriers.
5 CONCLUSION
We report the first two unrelated patients of Ashkenazi Jewish descent known to be affected with WABS, who are both homozygous for the same pathogenic variant: c.1763-1G>C. RNA studies show that this variant does affect splicing, which results in a frameshift of the open reading frame. These patient's phenotypes are consistent with the previously reported patients with WABS. Prior to this report, affected individuals were known to be of various ethnic groups with different pathogenic variants. Carrier screening analysis of Orthodox Jewish individuals through the Dor Yeshorim screening program revealed the carrier frequency to be 1 in 68 in the Ashkenazi Jewish population. We hypothesize a high rate of spontaneous abortions of affected fetuses and/or subfertility among carrier couples. Further studies are required to investigate this hypothesis. Additional carrier screening studies should also be performed to confirm the carrier frequency in the Ashkenazi Jewish population in more diverse Jewish populations. If additional studies also suggest a high carrier frequency, we suggest including DDX11 to Ashkenazi Jewish carrier screening panels.
ACKNOWLEDGMENTS
The authors would like to thank the patients and their families.
CONFLICT OF INTEREST
None.




