Novel phenotype of achondroplasia due to biallelic FGFR3 pathogenic variants
Funding information: National Institutes of Health, Grant/Award Number: T32GM007454
Abstract
Pathogenic variants in the fibroblast growth factor receptor 3 (FGFR3) gene are responsible for a broad spectrum of skeletal dysplasias, including achondroplasia (ACH). The classic phenotype of ACH is caused by two highly prevalent mutations, c.1138G > A and c.1138G > C (p.Gly380Arg). In the homozygous state, these variant results in a severe skeletal dysplasia, neurologic deficits, and early demise from respiratory insufficiency. Although homozygous biallelic mutations have been reported in patients with ACH in combination with hypochondroplasia or other dominant skeletal dysplasias, thus far, no cases of heterozygous biallelic pathogenic ACH-related variants in FGFR3 have been reported. We describe a novel phenotype of an infant with two ACH-related mutations in FGFR3, p.Gly380Arg and p.Ser344Cys. Discordant features from classic ACH include atypical radiographic findings, severe obstructive sleep apnea, and focal, migrating seizures. We also report the long-term clinical course of her father, who harbors the p.Ser344Cys mutation that has only been reported once previously in a Japanese patient. The phenotype of heterozygous biallelic mutations in FGFR3 associated with ACH is variable, underscoring the importance of recognition and accurate diagnosis to ensure appropriate management.
1 INTRODUCTION
Pathogenic variants in the fibroblast growth factor receptor 3 (FGFR3) gene underlie a broad spectrum of skeletal dysplasias. These encompass hypochondroplasia (HCH, OMIM #146000), achondroplasia (ACH, OMIM #100800), severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN, OMIM# 616482), and the lethal thanatophoric dysplasia (TD, OMIM# 187600) (Bonaventure et al., 1996; Martínez-Frías, de Frutos, Bermejo, Nieto, & ECEMC Working Group, 2010; Rousseau et al., 1994; Tavormina et al., 1999, 1995).
Almost all ACH patients have one of two common mutations, c.1138G > A or c.1138G > C (p. Gly380Arg). These mutations constitutively activate the FGFR3 receptor, causing abnormal membranous ossification, suppressing chondrocyte growth and proliferation, and ultimately hindering bone elongation (Bellus et al., 1995; Sahni et al., 1999; Shiang et al., 1994; Su et al., 1997). Homozygous p. Gly380Arg variants lead to a lethal skeletal dysplasia with a reduced thoracic cage, neurologic deficits from CNS anomalies, cervicomedullary stenosis, and stillbirth or neonatal demise from respiratory insufficiency (Stanescu, Stanescu, & Maroteaux, 1990).
Biallelic variants in FGFR3 causative for ACH and HCH have been reported in several patients with a milder skeletal phenotype (Chitayat et al., 1999; Couser et al., 2017; Flynn & Pauli, 2003; Huggins et al., 1999; Sommer, Young-Wee, Frye, & Reynolds, 1987) than homozygous ACH or coinheritance of mutations in genes causative for ACH and another dominant skeletal dysplasia, which usually presents with neonatal death due to respiratory insufficiency (Unger et al., 2001; Young, Ruggins, Somers, Zuccollo, & Rutter, 1992). We report a patient with heterozygous biallelic ACH-related variants in FGFR3, p.Gly380Arg, and p.Ser344Cys, manifesting a skeletal dysplasia more severe than classic ACH, accompanied by restrictive lung disease, severe obstructive sleep apnea, and seizures. We also report the clinical outcome of her father, who harbors the rare p.Ser344Cys variant that has only been reported once in a Japanese infant.
2 CLINICAL REPORT
Patient 1: The female index case was suspected to have a skeletal dysplasia on second trimester ultrasound scanning due to findings of shortened long bones and deceleration of linear growth, ascribed to ACH given the family history of both parents with ACH. No other prenatal abnormalities were observed and no prenatal testing was undertaken. She was born at 38 weeks 4 days gestation via cesarean section for breech presentation and maternal pelvic insufficiency due to ACH.
Family history is significant for the 25-year-old mother with classic ACH and the p.Gly380Arg mutation and the 29-year-old father with atypical ACH and the p.Ser344Cys mutation. The family is of mixed European heritage and there is no known consanguinity.
At birth, the proband manifested frontal bossing, wide-open fontanelles, midface hypoplasia, micrognathia, trident hands with brachydactyly, and redundant skin folds, although her phenotype appeared more severe than classic ACH. Birth weight was 2.88 kg (20th percentile), length 44 cm (0.7th percentile, −5SD), and head circumference 37.0 cm (50th percentile) on ACH charts (Hoover-Fong, McGready, Schulze, Barnes, & Scott, 2007; Horton, Rotter, Rimoin, Scott, & Hall, 1978). She had severe rhizomelic shortening of the upper and lower limbs, a small, bell-shaped chest, limited elbow extension and marked hypermobility of her fingers, knees, and ankles (Figure 1a,b). There was no hepatosplenomegaly or acanthosis nigricans. Sequencing of FGFR3 revealed two pathogenic variants in trans; c.1138G > C (p.Gly380Arg) and c.1031C > G (p.Ser344Cys). The latter is not present in population databases and in silico scores predict pathogenicity, and is classified as 3P by the ACMG-AMP Sherloc criteria (Nykamp et al., 2017).
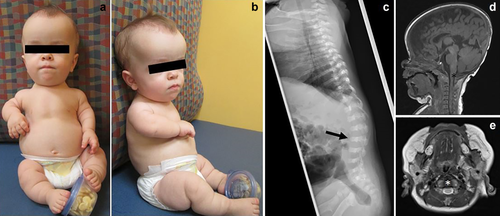
Early imaging of proband. (a,b) Frontal and profile view of patient 1. (c) Skeletal survey at age 3 weeks. Atypical radiographic findings for classic ACH demonstrated are moderate platyspondyly (arrow) and short AP diameter of the ribs. (d,e) MRI brain and upper cervical spine at age 2 months. Sagittal T1 (d) and axial T2 at level of upper cervical cord (e) images show diffuse moderate cervical canal stenosis and narrowing of the foramen magnum (asterisks). T1 hypointense and T2 hyperintense CSF is noted around the upper cervical cord. No cord compression or cord signal abnormality [Color figure can be viewed at wileyonlinelibrary.com]
In addition to classic radiographic findings of ACH, a skeletal survey at 3 weeks of age revealed moderate platyspondyly and short AP diameter of the ribs (Figure 1c). At 2 months, she was hospitalized for apneic episodes and non-febrile seizures of unclear etiology. An EEG showed bilateral temporal lobe seizures, which were treated with levetiracetam. An MRI showed normal temporal lobes and no myelomalacia or structural abnormalities of the brain. Moderate cervical canal stenosis was noted, typical for ACH, without associated cord compression (Figure 1d,e). Polysomnography (PSG) showed moderate obstructive sleep apnea with an apnea-hypopnea index (AHI) of 34/hr (normal < 5/hr). Echocardiogram was normal. She was started on nighttime oxygen at 0.5 L/min. Repeat PSG at 18 months showed worsening sleep apnea with an increase of AHI to 94/hr. She underwent an adenotonsillectomy at 20 months resulting in mild improvement. Her thoracolumbar kyphosis worsened at age 18 months, with new anterior inferior beaking of L2, atypical for ACH (Figure 2a). MRI of the brain and cervical spine at 20 months for new-onset upper motor neuron signs showed further progression of cervical canal stenosis with new upper cervical myelomalacia, new inferior beaking of the cerebellar tonsils, and new mild to moderate lateral and third ventriculomegaly (Figure 2b–d), prompting cervical decompression at 22 months of age.
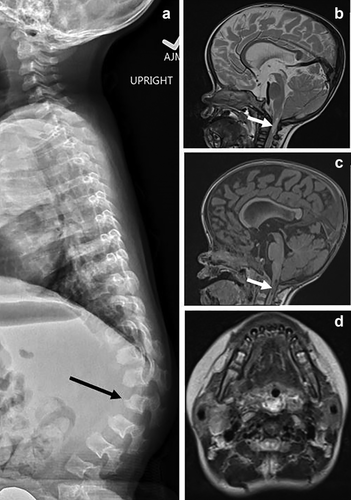
Follow up imaging of proband. (a) Thoracolumbar spine lateral radiograph at age 18 months. There is further accentuation of the thoracolumbar kyphosis around the L2 vertebra, which now has prominent anterior inferior vertebral body beaking (black arrow), which is also atypical of heterozygous ACH. Other thoracolumbar vertebrae demonstrate a bullet-shaped configuration of the vertebral bodies with increased posterior scalloping. (b–d) MRI brain and upper cervical spine at age 20 months. Sagittal T1 (b), sagittal T2 (c), and axial T2 (d) demonstrate new central T1 hypointense and T2 hyperintense signal in the upper cervical cord consistent with myelomalacia and reduced amount of CSF signal surrounding the upper cervical cord compared to baseline imaging (white arrows). There is new inferior pointing of the cerebellar tonsils. New mild to moderate lateral and third ventriculomegaly (not shown) was also demonstrated
Postsurgical PSG at 23 months showed significant improvement of her sleep apnea with an overall AHI of 7.2/hr. Her weight was 7.1 kg (<5th percentile, −2SD), length 59 cm (<5th percentile, −2SD), and head circumference 51.5 cm (20th percentile) on ACH charts. She had a normal neurologic examination and no cognitive or language delays but had delayed ACH-adjusted gross motor development as she was not sitting independently. She has bilateral mild sound field conductive hearing loss due to Eustachian tube dysfunction. She continues on levetiracetam although she has not had a seizure since 2 months of age and her MRI has normalized.
Patient 2: The proband's father was born to a mother of normal stature and he was given up for adoption at 6 months of age. Neonatal history was significant for failure to thrive requiring gastrostomy feedings and respiratory insufficiency requiring home oxygen therapy until 17 months of age. He continued on metaproterenol treatment for his ongoing respiratory difficulties. He was evaluated at 23 months of age for severe failure to thrive and difficulty breathing. His length was 63.3 cm (<5th percentile), weight was 5.42 kg (<5th percentile), and OFC was 63 cm (>95th percentile) on ACH charts. He manifested marked hypotonia, generalized decreased muscle mass, diminished deep tendon reflexes, and limited extension of both elbows. He had a narrow skull base and a wide-open anterior fontanelle. He lacked midface hypoplasia and rhizomelia. Genetic testing for the common FGFR3 variants was negative. Central sleep apnea prompted CPAP around age 7–8 years, and he continues presently. He had severe thoracolumbar kyphosis requiring surgical correction at 9 years of age, which was complicated by hardware failure and osteomyelitis of the spine. Postsurgical MRI showed marked wedging of the T10 vertebral body, and less severe wedging of the contiguous vertebrae. He did not have any social or intellectual delays, seizures, hearing loss, or hydrocephalus. At 30 years of age, he has rhizomelic shortening of all extremities without marked midface hypoplasia, frontal bossing, or acanthosis nigricans. Although he has atlantoaxial instability, this has not warranted surgical intervention. Aside from nocturnal respiratory support, he remains in good health and is gainfully employed as a teacher.
3 DISCUSSION
Herein, we report the outcome of heterozygous biallelic pathogenic FGFR3 variants in a patient with a novel ACH-plus phenotype. The common ACH p.Gly380Arg mutation and a much rarer ACH-related p.Ser344Cys FGFR3 variant, which was previously reported as pathogenic for ACH and not HCH, coexist in this patient as the result of inheritance from her ACH-affected parents. In contrast to homozygous p.Gly380Arg mutations, this combination has not resulted in a lethal outcome.
The rarer paternally inherited FGFR3 variant p.Ser344Cys has been recently reported as pathogenic in an infant case of heterozygous ACH manifesting as distinctive severe platyspondyly (Takagi et al., 2015). Similarly, our proband (III.1 in Supporting Information Figure 1) has severe platyspondyly atypical for classic ACH, and her father's (Supporting Information II.1) spinal imaging also described contiguous platyspondyly, suggesting a specific genotype-phenotype correlation. Although obstructive apnea-hypopnea is common in children with ACH (Tenconi et al., 2017), both of our patients (Supporting Information III.1 and II.1) experienced atypical recurrent apneic spells in infancy. The similarity to the previous case (Takagi et al., 2015) suggests this may be a recurrent outcome associated with the p.Ser344Cys mutation, although further cases are needed to confirm this.
It is likely that the severity of vertebral flattening and smaller chest in patients with this particular mutation predisposes to a more severe restrictive lung disease than classic ACH. There may be an increased risk for additional respiratory complications, since Supporting Information III.1 has persistent moderate sleep apnea despite adenotonsillectomy and cervical decompression. None of the patients with the p.Ser344Cys mutation reported thus far (N = 3 including present two cases, all heterozygous) have cognitive delays, acanthosis nigricans, or long bone bowing typically seen in patients with other severe dominant FGFR3-related skeletal dysplasias (Zankl et al., 2008).
Whether the non-febrile seizure seen in our proband (Supporting Information III.1) is attributable to her FGFR3 mutations, apnea-induced hypoxia, or other causes remains to be elucidated. Seizures associated with temporal lobe dysplasia have been described in homozygous p.Gly380Arg patients and other severe forms of FGFR3-related skeletal dysplasias (Kannu & Aftimos, 2007; Philpott et al., 2013; Romeo et al., 2014; Zankl et al., 2008). However, our proband lacks any structural brain abnormalities. Patients with seizures and temporal lobe abnormalities all had heterozygous mutations within the transmembrane or tyrosine kinase domains of FGFR3, which is demonstrated in murine models to increase cortical progenitor proliferation and decrease apoptosis during cortical brain development, presumably predisposing to temporal lobe dysplasia, and propensity to develop seizures (Inglis-Broadgate et al., 2005; Thomson, Pellicano, & Iwata, 2006). The p.Ser344Cys mutation is located in the highly conserved IgIII domain of FGFR3 as opposed to the transmembrane location of the common p.Gly380Arg mutation (Supporting Information Figure 2), which may explain the absence of temporal lobe abnormalities and intellectual delay in our proband and her father.
Molecular confirmation in clinically and radiographically assigned ACH by targeted p.Gly380Arg mutation testing varies according to individual clinician decision and is often not undertaken due to the assumption that the diagnosis is correct and the result would not change medical management. No formal molecular testing guidelines exist for ACH, although expert opinion suggests full sequencing of FGFR3 if clinical diagnosis is uncertain, atypical features are present, or similar skeletal dysplasia conditions are suspected. Our report illustrates the importance of an accurate molecular diagnosis in patients with ACH with atypical features. Ongoing discoveries of novel FGFR3 variants are revealing that certain genotypes may have important implications for health outcomes early in life such as a more severe restrictive respiratory disease than otherwise expected for classic forms of ACH (Xue et al., 2014). Rare variants in FGFR3 may occur with greater prevalence than is currently understood. We conclude that DNA sequencing of all exons of FGFR3 should be considered in ACH patients with atypical features, particularly vertebral differences such as platyspondyly, severe apnea or restrictive lung disease, worsening cervical canal stenosis, seizures, or parents with a skeletal dysplasia. Better delineation of the various ACH phenotypes and possible correlation with genotypes are required to guide individualized patient management.
ACKNOWLEDGMENT
We thank the family for allowing us to report this case.
CONFLICTS OF INTEREST
The authors declare that they have no relevant financial conflicts of interests.