Further delineation of the GDF6 related multiple synostoses syndrome
Abstract
A mutation in GDF6 was recently found to underlie a multiple synostoses syndrome. In this report, we describe the second family with GDF6-related multiple synostoses syndrome (SYNS4), caused by a novel c.1287C>A/p.Ser429Arg mutation in GDF6. In addition to synostoses of carpal and/or tarsal bones, at least 6 of 10 affected patients in this family have been diagnosed with mild to moderate hearing loss. In four of them otosclerosis was said to be present, one patient had hearing loss due to severe stapes fixation at the age of 6 years, providing evidence that hearing loss in the GDF6-related multiple synostoses syndrome can be present in childhood. Two others had surgery for stapes fixation at adult age. We hypothesize that, identical to the recently published GDF6-related multiple synostoses family, the p.Ser429Arg mutation also leads to a gain of function. The previously reported c.1330T>A/pTyr444Asn mutation was located in a predicted Noggin and receptor I interacting domain and the gain of function was partly due to resistance of the mutant GDF6 to the BMP-inhibitor Noggin. The results in our family show that mutations predicting to affect the type II receptor interface can lead to a similar phenotype and that otosclerosis presenting in childhood can be part of the GDF6-related multiple synostoses syndrome.
1 INTRODUCTION
Multiple synostoses syndromes are a group of related disorders, characterized by progressive symphalangism and/or multiple joint fusions in hands and feet. Other joints, for example, elbows, hips, or vertebrae, can also be affected. In some families, brachydactyly, conductive hearing loss or specific facial features are noted (Potti, Petty, & Lesperance, 2011). To date, several molecular subtypes have been identified, with mutations in genes involved in bone morphogenetic protein (BMP) signaling, including the gene encoding the BMP antagonist NOG (SYM1A, OMIM #185800, SYNS1, OMIM #186500, and TCC #186570), GDF5 (SYNS2, OMIM #610017, SYM1B, OMIM #615298), or FGF9 (SYNS3, OMIM #612961). These multiple synostoses syndromes are characterized by carpal and tarsal fusions, symphalangism, humeroradial synostoses, vertebral fusions, and dependent on subtype characteristic facial features (SYNS1, SYNS2) and progressive conductive hearing loss (SYNS2) (Dawson et al., 2006; Takahashi et al., 2001; Wu et al., 2009). Several in vitro and animal studies showed that the common denominator was increased BMP signaling, caused by loss of function, resistance to inhibition by Noggin or attenuation of the BMP pathway due to reduced FGF9 signaling (Gong et al., 1999; Groppe et al., 2002; Schwaerzer et al., 2012; Seemann et al., 2009; Takahashi et al., 2001; Wu et al., 2009).
Recently, a gain of function mutation in a related member of the BMP family, the GDF6 (growth and differentiation factor 6) gene, was identified in a new subtype of multiple synostoses syndrome (SYNS4) (Wang et al., 2016). GDF6 is a member of the bone morphogenetic protein (BMP) sub-family of transforming growth factor-beta (TGFß) signaling ligands. Binding of GDF6 to the extracellular domains of type I and type II TGFß receptors leads to heterodimeric receptor complex formation and downstream activation of the Smad 1/5/8 pathway. This signaling pathway has been shown to play an important role in skeleto- and organogenesis (Yadin, Knaus, & Mueller, 2016). We identified a second family with a phenotypically highly overlapping multiple synostoses syndrome and in addition hearing loss caused by otosclerosis due to a novel GDF6 mutation.
2 MATERIALS AND METHODS
Physical examination was performed in patient II-2, III-2, III-5, IV-1, and IV-2. In four patients, patient II-2, III-2, IV-1, and IV-2 radiographs of the hands and feet were available, radiographs of the cervical spine in II-2, III-2, and IV-1. Patient II-2, III-2, and IV-1 had ophthalmological investigations. Information about hearing tests was obtained. Patient II-2 gave informed consent to perform DNA analysis in the NOG, GDF5, and GDF6 gene. After identification of a mutation in GDF6 in patient II-2, the parents of patient IV-1 and IV-2 gave consent to test this GDF6 mutation in their affected child. Mutation analysis of the coding exons and exon/intron boundaries of GDF5, GDF6, and NOG was performed by Sanger sequence analysis (primers available on request).
3 RESULTS
3.1 Clinical data
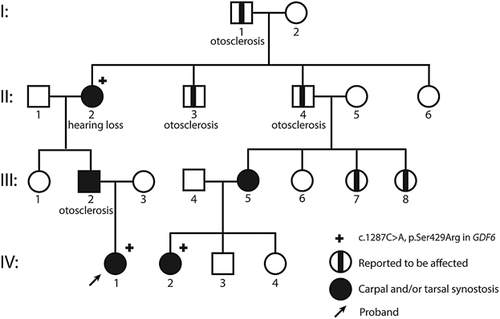
We present a four-generation family affected by an autosomal dominant multiple synostoses syndrome leading to tarsal and carpal bone fusions and variable hearing loss (Figure 1). We studied five affected individuals by physical and four by radiological examination (clinical information is presented in Supplemental Table S1).
The proband (IV-1) was a 7-year old female who presented with habitual toe walking, for which she underwent a bilateral achilles tendon release in the past. Physical examination showed broad forefeet with overlapping toes and bilateral mild diminished dorsal and plantar flexion in the talocrural joint. The hands showed bilateral clinodactyly of digiti V. Stature, intelligence, and mobility of other joints were normal. Radiographs of the hands showed fusion of os hamate, os triquetrum, and os lunate, in addition fusion of os capitate and os trapezoid, and hypoplasia of midphalanx of digitus V. In the feet bilateral fusion of os cuneiform intermedius and cuneiform laterale with metatarsal II and III, bilateral fusion of os cuboideum with metatarsal IV and V and fusion of the talus with the navicular bone was noted (Figure 2, Supplemental Table S1). Ophthalmological examination was normal.
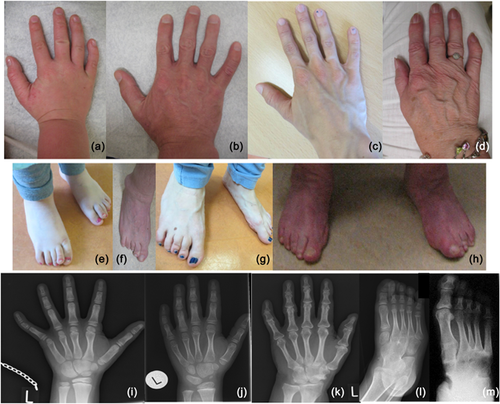
Other examined family members (IV-2, III-2, III-5, II-2) also had variable diminished ankle and foot mobility resulting in limited ability to walk long distances and accompanied by pain. Individual II-2 also had problems with standing. All showed broad forefeet with overlapping toes and flat arches. There were no functional limitations of the hands or elbows. Several family members had clinodactyly of digitus V or brachydactyly. No dysmorphic facial features were noted during physical examination of patient IV-1, IV-2, III-2, III-5, and II-2. Radiological examination showed variable fusion of carpal bones and fusion of tarsal bones (Figure 2, Supplemental Table S1). Radiological examination of the cervical spine was performed in three patients and found to be normal in patient IV-1 and III-2. In patient II-2, aged 65 years, degenerative abnormalities were present in uncovertebral joints and intervertebral joints C5-C7 with decrease of joint space.
Individuals I-1, II-3, and II-4 were said to be affected by multiple synostoses and otosclerosis since childhood. Individuals I-1 and II-4 underwent stapes surgery in their late forties, respectively, 64 years. Medical data were however not available. Individual III-2 was diagnosed with a congenital stapes fixation at age 6 years. At age 15 years bilateral stapes surgery was performed, successfully. Individual II-2 had in the right ear a mixed hearing loss in the high frequencies with a fletcher index of 37 dB and an air bone gap of 20dB-40 db in the high frequencies. The left ear showed a mixed hearing loss in the high frequencies as well with a fletcher index of 30 dB and an air bone gap of 10-40 dB. CT of the mastoid was normal. At the age of 8 years patient IV-1 had conductive hearing loss in the right ear with a fletcher index of 20 dB which can be explained by the negative pressure in her middle ear (shown with tympanometry). The left ear showed a mainly perceptive cookie bite hearing loss of 18 dB in the fletcher index. Individual III-5 also reported mild hearing loss, but pure tone audiometry at the age of 28 years was normal.
DNA analysis of two multiple synostoses genes (NOG, GDF5) in patient II-2 showed normal sequence data. Based on animal models and the publication by Wang et al. (2016) we hypothesized that a mutation in GDF6 could be responsible for the phenotype (Rountree, Sinha, Thacker, Higgins, & Kingsley, 2003). Indeed DNA analysis of GDF6 showed the c.1287C>A/p.Ser429Arg variant which co-segregated in the family; family member IV-1 and IV-2 were tested and found to be carrier of the mutation. As a consequence, family members III-2, II-4, and III-5 are obligate carriers of the mutation. The serine at codon 429 is a highly conserved amino acid residue in the C-terminal transforming growth factor-beta domain, conserved up to C.elegans (Supplemental Figure S1). The c.1287C>A/p.Ser429Arg variant is predicted to be pathogenic by Align GVGD, SIFT, and Mutation Taster and has not been observed previously in Exac or GoNL (Francioli et al., 2014; Lek et al., 2016).
4 DISCUSSION
Here, we report the second family with multiple synostoses due to a missense mutation in the TGFß mature domain of GDF6, confirming the previous identification of a fourth locus for multiple synostoses syndrome (Wang et al., 2016). We consider the p.Ser429Arg mutation as pathogenic based on the conservation of the amino acid, results of prediction programs, co-segregation in the family and phenotypic similarities with the first described family.
The skeletal phenotype with variable carpal and tarsal synostoses but (in contrast to SYNS1, SYNS2, and SYNS3) lacking symphalangism, humeroradial synostoses or vertebral fusions, is highly similar to the phenotype in the first reported family with SYNS4. Furthermore, both our family and the first reported family with SYNS4 lack dysmorphic facial features previously reported in other multiple synostoses syndromes (Higashi & Inoue, 1983; Wang et al., 2016).
The hearing problems however differ. In the first reported family progressive conductive hearing loss after the age of 40 years was found in affected patients, while in our family severe hearing problems already occurred in childhood and could be the result of congenital or progressive stapes fixation/otosclerosis. This is in line with studies in mice which have shown that GDF6 plays an important role in cartilage development and proliferation in the joints of the middle ear (Settle et al., 2003).
GDF6 is a member of the TGFß/BMP family of ligands. These ligands form homo- or heterodimers which, by assembling a heteromeric complex consisting of type I and type II receptors, lead to activation of Smad 1/5/8 signaling pathway (Yadin et al., 2016). An important role has been established in skeletogenesis and joint formation for GDF6 and its homologue GDF5, both in human and in animal models (Seemann et al., 2009; Settle et al., 2003).
Based on studies of its homologue, GDF5, the p.Ser429Arg mutation in our family is predicted to affect the type II receptor interface, while the previously reported p.Tyr444Asn mutation is in the region critical for binding type I receptor and the BMP antagonist noggin (Seemann et al., 2009; Wang et al., 2016). Functional studies in the family with the p.Tyr444Asn showed that the gain of function of the mutation was partly due to resistance of the mutant GDF6 to the BMP-inhibitor Noggin (Wang et al., 2016). Previously, the homologous p.Ser475Asn mutation located in the type II receptor interface in GDF5 was shown to cause SYNS2. In vitro studies showed a lower affinity of this mutated protein with the type II receptor and thereby diminished stimulation of the Smad and p38 MAPK pathway. More importantly the protein was also shown to have a lower affinity to the BMP/GDF antagonist Noggin leading to increased endochondral ossification and diminished joint formation (Schwaerzer et al., 2012). Based on the gain of function effects described for these previously reported GDF5 and GDF6 mutation, we hypothesize that the p.Ser429Arg mutation in our family causes multiple synostoses by resistance to inhibition by Noggin as well.
Missense GDF6 mutations have also been reported in patients with a wide spectrum of congenital anomalies other than SYNS4 including Klippel-Feil syndrome and microphthalmia or coloboma (Supplemental Table S2) (Asai-Coakwell et al., 2009; den Hollander et al., 2010; Slavotinek et al., 2015; Tassabehji et al., 2008; Ye et al., 2010). However, these missense mutations do not seem to represent monogenic syndromes with full penetrance, since several mutations were detected in unaffected parents or did not co-segregate with the phenotype in the family (Asai-Coakwell et al., 2009; den Hollander et al., 2010). In contrast to the two GDF6 missense mutations associated with SYNS4 the majority of these missense mutations is frequently detected in normal control populations (Supplemental Table S2). Further studies are needed to elucidate the contribution of GDF6 missense mutations in the etiology of congenital anomalies other than multiple synostoses syndrome.
In conclusion, in this short report we describe the second family with type four multiple synostoses syndrome (SYNS4) due to a missense mutation in the mature TGFß domain of GDF6. Features encompass carpal and tarsal synostosis and hearing loss. This study shows that the hearing loss may be due to otosclerosis, sometimes already presenting in early childhood with stapes fixation, emphasizing the role of GDF6 in maintenance of middle ear joints. In addition, this study provides evidence that, similar to GDF5 related multiple synostoses syndrome, not only mutations in the NOG and receptor I interface area, but also in other parts of the protein like the receptor II interacting domain, can be involved in causing this highly penetrant disorder.
ACKNOWLEDGMENTS
We thank the patients and their parents for their co-operation. We thank Glen Monroe for making Figure 1.