Left ventricular noncompaction cardiomyopathy in a patient with trisomy 13: A report and review of the literature
Abstract
Left ventricular noncompaction cardiomyopathy (LVNC) is characterized by prominent trabecular meshwork, and it is thought to result from arrest of the normal compaction process during embryogenesis. Patients with LVNC may be asymptomatic or have symptoms ranging from heart failure to stroke, life-threatening arrhythmias, or sudden death. The frequency of LVNC in children has increased with longer clinical courses. About 80% of patients with trisomy 13 have a congenital cardiac abnormality, but a clinical description of LVNC with trisomy 13 is lacking because of its poor prognosis and lack of awareness about LVNC. We described a patient with trisomy 13 who was diagnosed with LVNC-dilated phenotype and died suddenly, as well as two additional patients with LVNC. All three patients had chronic heart failure without congenital heart disease and were treated with diuretics. To manage trisomy 13 with or without congenital heart disease, cardiac disease such as LVNC may present at any ages, and therefore cardiac evaluation should be considered as a part of their appropriate management.
1 INTRODUCTION
Left ventricular noncompaction cardiomyopathy (LVNC) is rare and characterized by bilayered myocardium consisting of prominent ventricular trabeculae, and deep intertrabecular recesses. LVNC was first described as the postnatal persistence of spongy myocardium in 1975 (Dusek, Ostadal, & Duskova, 1975). Although LVNC is frequently misdiagnosed as dilated or hypertrophic cardiomyopathy, it is classified as a distinct cardiomyopathy by the American Heart Association (Maron et al., 2006). Previous reports have suggested that LVNC is associated with Barth syndrome, mitochondrial diseases, myotonic dystrophy, and other genetic disorders such as LDB3/ZASP, Lamin A/C, MYH7 (Rooms, Dujardin, & Sutter, 2015). It is hypothesized that LVNC results from a failure of the normal compaction process during embryogenesis. Patients present clinically along a broad spectrum from no symptoms to heart failure, stroke, life-threatening arrhythmias, or sudden death. LVNC has several types such as benign, dilated, hypertrophic, hypertrophic dilated, restrictive, right ventiricular or biventricular, and with congenital heart disease. The dilated subtype of LVNC is characterized by concomitant left ventricular dilation and systolic dysfunction. (Towbin, Lorts, & Jefferies, 2015). The diagnosis of LVNC is often made by echocardiography. LVNC remains a diagnostic and management challenge because of the varied manifestations of overlapping cardiomyopathies, and the lack of precise diagnostic tools and criteria (Paterick & Tajik, 2012).
About 80% of patients with trisomy 13 present with a congenital cardiac abnormality, including ventricular septal defect, patent ductus arteriosus, and atrial septal defect, less frequently (less than 50%) anomalous pulmonary venous return, overriding aorta, pulmonary stenosis, hypoplastic aorta, atretic mitral and aortic valves, and bicuspid aortic valve (Jones, 2015). These congenital heart diseases have been well-described (Carey, 2010), but a search of the literature using PubMed found only two patients with trisomy 13 and LVNC.
We describe the detailed manifestation of LVNC with trisomy 13 and two prior case reports (McMahon et al., 2005; Monden, Shiraishi, Ichida, & Momoi, 2011). It is important that systolic function should be examined by cardiovascular specialists when patients with trisomy 13 present signs of systolic dysfunction, even in those without congenital heart diseases.
2 CASE REPORTS
A 3-year and 8-month-old boy had been hospitalized because of wheezing and tachypnea due to bronchial asthma attack, which was later found to be pulmonary edema. He had been diagnosed with full trisomy 13 by chromosomal analysis during the neonatal period. He had polydactyly, glaucoma, inguinal hernia, an omphalocele that was cured by surgery during the neonatal period, a ventriculoperitoneal shunt for congenital hydrocephalus, daily breath-holding spells and convulsions, asthma and severe motor, and mental developmental delay; he also required nasogastric feeding. In the neonatal period, congenital heart disease and cardiomyopathy were not detected by echocardiography by cardiovascular specialists, and the presence of LVNC was not identified retrospectively. His systolic function had not been monitored since then. There was neither family history of cardiomyopathy nor symptoms of cardiovascular disease.
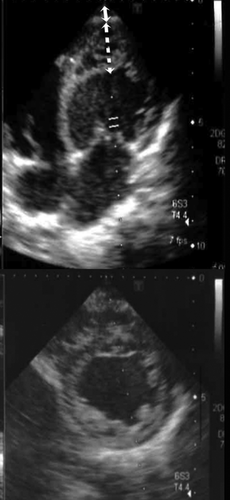
On admission, his vital signs were: body temperature 36.8°C; respiratory rate 33 breaths per min; and pulse rate 120 beats per min. Because the oxygen saturation was decreased to 92% on room air, oxygen inhalation was started. Treatment for the asthma included intravenous prednisolone and β-agonist inhalation. However, his respiratory distress did not improve, and edema developed on his face and extremities. Three days after admission, cardiovascular examination revealed a gallop murmur and bilateral crackles over the lungs. His chest radiograph showed cardiomegaly with a cardiothoracic ratio of 58% and pulmonary edema. A transthoracic two-dimensional echocardiogram demonstrated left ventricular dilation, trivial mitral regurgitation, prominent trabeculations and deep recesses within the apex, and a left ventricular ejection fraction of 43.3% (Figure 1). The non-compacted to compacted segment ratio was 3.0 (normal range <2.0). The electrocardiogram showed sinus rhythm, marked ventricular biventricular hypertrophy with extreme QRS complex voltage, and a diffuse flat T-wave. The chemistry profile showed an increased BNP to 306.0 pg/ml (normal range <18.4 pg/ml). He was diagnosed with LVNC-dilated phenotype, and the systolic dysfunction caused acute heart failure. He was treated with intravenous diuretics and then continued to have oral diuretics (Lasix® 2 mg/kg/day and Aldactone A® 2 mg/kg/day), and an angiotensin converting enzyme inhibitor (Enalapril maleate® 0.08 mg/kg/day). The edema and tachypnea disappeared. He was discharged home about a month later. Subsequently, he had relapses and remissions of acute exacerbations with chronic heart failure, and was admitted to the hospital ten times in a year. He gained about 1 kg of weight by edema and presented tachypnea. Intravenous diuretics were maintained during hospitalization. A β-blocker was added and then increased (Lopresor® 0.5 mg/kg/day). He discharged shortly after the hospital treatment to allow his family to spend time with him. For cardiogenic pulmonary edema, non-invasive positive pressure ventilation had been used since the third admission. It reduced the work of breathing and left ventricular preload, and decreased the heart rate by causing pulmonary hyperinflation. His tachypnea had disappeared, however the left ventricular ejection fraction decreased to 32.0%, and the serum BNP increased to 909.0 pg/ml at the peak. No intracardiac thrombi were detected on a regular echocardiogram. The electrocardiogram showed sinus rhythm during hospitalization. After the tenth discharge, at age 4 years and 8 months old, he died suddenly at home while sleeping with an artificial ventilator following a bath. There were no signs of decompensation, such as edema or tachypnea. The ventilator documented a hypoventilation alarm, which might suggest sudden cardiopulmonary arrest.
3 DISCUSSION
LVNC has been recognized as a distinct entity of cardiomyopathy with a genetic correlation (Maron et al., 2006). Genetic syndromes with LVNC include Beals, CHARGE, Marfan, Noonam, Sotos, trisomy 13 and 18, 22q11.2 deletion, and others (Shieh, 2013). Several echocardiographic criteria for the diagnosis of LVNC have been proposed, such as a noncompacted to compacted segment ratio >2. According to serial echocardiographic studies, LVNC was not diagnosed initially but became evident on subsequent examination (Floria, Tinica, & Grecu, 2014). The frequency of LVNC in children has increased due to improved awareness and advanced diagnostic tools. Medical treatment depends on associated comorbidities and underlying diseases. Some patients undergo orthotopic heart transplantation (Pignatelli et al., 2003). The prognosis of patients with LVNC is determined by the degree and progression of heart failure, and the presence of thromboembolic events and arrhythmias. In this report, the patient had already presented with severe heart failure at the time of diagnosis of LVNC, and his heart function was deteriorating with repeated acute exacerbation over a year; he died suddenly at home.
The association of LVNC with trisomy 13 has rarely been reported. There were only two girls reported with a diagnosis of LVNC (Table 1) (McMahon et al., 2005; Monden et al., 2011). One of them had genetic analysis for TAZ and LDB3, and they were not identified. The ages at diagnosis were 11 years and 9 years. They had no underlying congenital heart disease, and presented with edema and tachypnea at the onset. In comparison of them and the present patient, his associated features and clinical course of LVNC were more severe, and the ages of onset was younger.
| Case report | Genetic analysis | Sex | Age at diagnosis (year) | Associated features | Treatment | Outcome |
|---|---|---|---|---|---|---|
| McMahon et al. (2005) | Trisomy 13 | Female | 11 | Microcephaly | Diuretics | Alive |
| Overlapping fingers | ||||||
| Bilateral blindness | ||||||
| Non-insulin-dependent diabetes | ||||||
| Asthma | ||||||
| Monden et al. (2011) | Trisomy 13 | Female | 9 | Cleft palate | Diuretics | Alive |
| Overlapping fingers | Angiotensin converting enzyme inhibitor | |||||
| Epilepsy | ||||||
| Present patient | Trisomy 13 | Male | 3 | Hydrocephaly | Diuretics | Dead a year after diagnose of LVNC |
| Omphalocele | Angiotensin converting enzyme inhibitor | |||||
| Inguinal hernia | β-blocker | |||||
| Hydrocele testis | ||||||
| Congenital glaucoma | ||||||
| Polydactyly | ||||||
| Epilepsy | ||||||
| Breath-holding spells | ||||||
| Asthma |
LVNC may be a rare cardiac complication of trisomy 13, havingnoted previously as chromosomal syndrome association with LVNC by Towbin et al. (2015), although there are currently no genetic loci on chromosome 13 associated with LVNC. In this patient, no additional genetic testing was performed, but we would advise a multi-gene cardiomyopathy panel for future patients (Shieh, 2013) which would clarify a potential additional genetic association between trisomy 13 and LVNC. People with trisomy 13 have limited activity and spend most of their time at rest because of their developmental handicaps. This may result in the manifestations of LVNC being missed or misdiagnosed as bronchial asthma, as in the present case. In addition, detection of subjective complaints of heart failure such as fatigue, palpitations, or dyspnea may also be delayed.
In conclusion, we advise a meticulous echocardiographicevaluation of the myocardium, in addition to cardiac structure to determine if LVNC may be more common in trisomy 13 than previously recognized.