Unclassifiable pattern of hypopigmentation in a patient with mosaic partial 12p tetrasomy without Pallister–Killian syndrome
Abstract
Pallister–Killian syndrome (PKS-#OMIM601803) is a multisystem developmental disorder typically due to the presence of an aneuploidy cell line, consisting of a supernumerary tetrasomic chromosomal marker (SCM) arisen from the short arm of chromosome 12 (12p isochromosome). The clinical phenotype, which is strictly related to the percentage and tissue distribution of aneuploid cells, is characterized by craniofacial dysmorphisms, pigmentary skin anomalies, limb shortening, congenital heart defects, diaphragmatic hernia, hypotonia, intellectual disability, and epilepsy. We report on a 4 year-old girl harboring a 12p partial isochromosome, involving the PKS critical region, affecting about 70% of circulating lymphocytes, urine, and saliva cells and fibroblast from a hyperpigmented skin spot, and 100% of fibroblasts from a hypopigmented skin spot. Interestingly, despite the high proportion of affected cells this patient did not present with PKS, and a pattern of linear and patchy pigmentary mosaicism was the sole clinical manifestation. The present observation suggests that partial 12p SCM can also result in mild phenotypes, and its prevalence in the human population could have been underestimated. Accurate dermatologic evaluation could be a major handle for genetic testing.
1 INTRODUCTION
Genetic mosaicism is defined as the coexistence of two different cell populations at least, carrying distinct genotypes, resulting from a postzygotic mutation affecting one cell and its offspring. The size of the mutated population depends on the precocity of mutational event. The clinical outcome is highly variable depending on the percentage of abnormal cells and their tissue distribution. Skin manifestations of mosaicisms include cutaneous lesions, probably reflecting keratinocytes migration paths during embryonic development. Although the majority of genodermatoses are due to germinal mutations, a significant number of skin disorders results from mosaic events, including point mutations, deletions, insertions, and chromosomal structural, and numerical abnormalities (Hartmann, Hofmann, Hoehn, Broecker, & Hamm, 2004; Verghese, Newlin, Miller, & Burton, 1999; Vreeburg & van Steensel, 2012). Pallister–Killian syndrome (PKS-OMIM#601803) is a distinct disorder, due to a mosaic extra isochromosome 12p (tetrasomy 12p) and, in rare cases, 12p partial tetrasomy, 12p trisomy (Vermeesch et al., 2005), and 12p13.31 duplication (Izumi et al., 2012). Associated aneuploidies origin as a germ cell aberration and the mosaicism results from the postzygotic loss of the marker chromosome. This condition is usually diagnosed in fibroblast karyotype, because of its often selection against in lymphocyte culture.
PKS is a multisystem developmental disorder characterized by craniofacial dysmorphisms, pigmentary skin anomalies, limb shortening, congenital heart defects, diaphragmatic hernia, hypotonia, intellectual disability, and epilepsy (Wilkens et al., 2012). Skin symptoms consist of linear hyper- and hypo-pigmented lesions being sometimes reminiscent of Blaschko's lines, which may be visible only under the Wood's lamp. Skin anomalies occur in about 40% of these patients, resulting from the presence of supernumerary 12p in fibroblasts. Therefore, a dermatologic examination of the skin by Wood's lamp is advisable when a phenotype suggestive of PKS is present (Guareschi et al., 2007).
We report on a young girl, harboring a mosaic partial tetrasomy 12p in blood lymphocytes, presenting with skin dyschromia unassociated with other PKS features.
2 CLINICAL REPORT
The patient, a 4-year-old girl, the first of two sibs, was born from non-consanguineous healthy parents with an unremarkable family history. Pregnancy was grossly normal, with only a slight decrease of amniotic fluid in the last weeks of gestation. Delivery at term was spontaneous, with birth weight of 4260 g, length 52 cm, and OFC 34.5 cm. Apgar scores were 9 and 10 at 1 and 5 min.
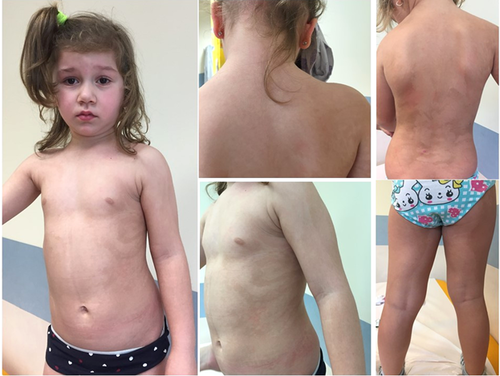
At the time of our evaluation, at age of 3 years and 10 months, the skin showed linear, patchy or vortex-like pigmentary disturbances only partially arranged along atypical lines of Blaschko without any apparent midline separation. The dermatologist described this features as an unclassifiable pattern of pigmentary mosaicism (Happle, 2014). The mother referred that the skin pattern was congenital and stable. Growth parameters were regular, within the upper normal ranges, a likely familiar trait (weight 21 kg, 75–90th centile; length 110 cm, 75–97th centile; OFC 51 cm, 75th centile). No significant dysmorphic feature was present, with the exception of depressed nasal bridge, mild nasal bulbous tip, and deep philtrum. No other ectodermal abnormality was present; hair and nails were normal (Figure 1). At 3 years of age, ocular evaluation disclosed a dishomogeneous pigmentation of fundus oculi, which was not confirmed 6 months later.
Psychomotor milestones were regularly achieved and neurological assessment was unremarkable.
3 MATERIALS AND METHODS
3.1 Cytogenetics
GTG-banding karyotype was performed according to standard procedures on metaphases obtained from PHA stimulated lymphocytes and on two different fibroblasts cultures: one from a hyper-pigmented cutis biopsy and the other from a hypo-pigmented fragment. Chromosome analysis of the patient and her parents was carried out at 550-band level.
3.2 Fish analysis
Multicolor cenM-FISH according to the manufacture protocol (XCyting Centromere Multi-Color Probe Mix, MetaSystems, Heidelberg, Germany) was carried out for analysing alfoid centromeric regions of all chromosomes.
Locus specific FISH analysis for the subtelomeric region of chromosome 12, was performed by means of ToTelVysion subtelomeric panel probes (Vysis Abbot-Molecular, IL).
3.3 Array-CGH
Array-CGH analysis was achieved using 4 × 180 ISCA oligo-array platform (Bluegnome, Cambridge, UK) on patient's DNA samples from peripheral blood, saliva, and urine cells. Assays were carried out according to the manufacturer's instructions and data analyzed by Bluegnome BluFuse Multi 4.2 software.
4 RESULTS
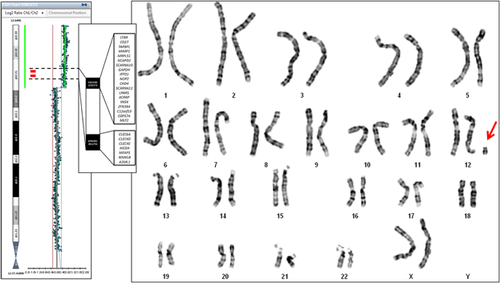
GTG-banded karyotype detected a 47,XX, + mar cell line, accounting for 70% of analyzed cells (Figure 2). CenM-FISH analysis was performed in order to identify the origin of the small supernumerary marker chromosome (SMC). The analysis did not detect any fluorescent signal on the SMC, suggesting that it did not contain alpha satellite repeats. Array-CGH test characterized the SMC as derivative from the distal portion of the short arm of chromosome 12 (Figure 2), 12p13.33p13.31, spanning until 9.7 Mb genomic position (h19). FISH analysis with 12pter locus specific subtelomeric probes confirmed the result showing a partial 12p tetrasomy (acentric inverted duplication marker). The duplicated region did not involve the centromere, consistently with the cenM-FISH result. Cytogenetic analyses on parental blood showed normal karyotypes. In order to assess the aneuploid cells distribution, skin biopsies from both the hyperpigmented and hypopigmented skin, urine, and saliva cells were collected. Karyotype analysis showed that the aneuploid cell population was present in about 70% of examined cells in the pigmented cutis fragment, a figure similar to that found in circulating lymphocytes, whereas it was found in all cells from the hypopigmented skin area. Array-CGH analysis disclosed the 12p isochromosome also in urine and saliva samples. The proportion of aneuploid cells was estimated in both tissues by array-CGH log2ratio calculation and it was considered as high as in blood sample (about 65%).
5 DISCUSSION
Failure in chromosomes segregation during cell division can result in karyotypically abnormal daughter cells, harboring aberrant chromosome number or structure.
Mosaicisms can be visible on the skin as hypo/hyper-pigmented streaks and spots, and the extent and type of the pattern is likely dependent on the precocity of the causal event during embryonic development. Very early events result in widespread variegation, whereas the late ones may cause changes to appear in only one sector, or even a small area within a sector (Happle, 2016; Lombillo & Sybert, 2005).
PKS is a rare chromosomal disorder, affecting less than 1 in 10,000 live-born. This disorder is characterized by global developmental delay, facial dysmorphisms, rhizomelic limb shortening, small hands and feet, nail hypoplasia, and pigmentary skin anomalies. Most cases result from mosaic supernumerary 12p isochromosome, which can be undetectable by karyotype blood analysis, because of aneuploid cells selection during lymphocyte cultures (Ballif et al., 2006). Presumably, full tetrasomy 12p would be a lethal condition which can result in live-born infants only in a mosaic state. Mosaic 12p trisomy also results in a similar phenotype. Izumi et al. (2012) have proposed 12p13.31 chromosome band as the syndrome critical region.
Only two cases of partial 12p tetrasomy have been described so far (Dufke et al., 2001; Vermeesch et al., 2005) in patients presenting with a disorder resembling PKS. Several mechanisms have been proposed for acentric marker chromosomes formation, such as inter- or intra-chromosomal U-type exchange during meiosis or mitosis (Voullaire et al., 2001), or through an intermediate hairpin molecule, promoted by the presence of an inverted repeat followed by an intra-molecule replication of the fragment end (Murmann et al., 2009). The acentric fragment is stabilized during cell division, by a neocentromere development, probably facilitated by epigenetic processes or reactivation of an ancestral centromere.
Here, we report the third patient affected by partial 12p tetrasomy mosaicism, the sole subject so far not associated with the full-blown PKS phenotype, as corroborated by the long-term follow-up by neurologists, ophthalmologists, and dermatologists.
Despite of the high percentage of aneuploid cells in different tissues (65–70% in blood, saliva, urine, and hyper-pigmented skin area; 100% in hypo-pigmented skin area) of the affected girl, evaluated at age of 4 years and 6 months, she presented dyschromia as the sole manifestation. Only minor nonspecific dysmorphic features were noted, including depressed nasal bridge, mild nasal bulbous tip, and deep philtrum. The acentric fragment encompassed the region previously described by Vermeesch et al. (2005) and involved the PKS critical region highlighted by Izumi et al. (2012). In published patients, psychomotor delay, cognitive impairment, dysmorphic features, ectodermal abnormalities with thin hair were reported. The mild phenotype in our proband, with normal psychomotor development, absence of significant dysmorphisms and exclusive cutaneous manifestation in the form of patchy or linear hypopigmentation, is likely due to the aneuploid cell distribution not affecting other critical tissues.
This case suggests that the partial 12p SCM mosaicism can manifest with a mild phenotype, distinct form classical PKS, and therefore, could have been underestimated. The present case underlines that an accurate dermatologic evaluation can be a handle for genetic testing. Detection of a partial 12 tetrasomy in prenatal diagnosis can be challenging in terms of predicting postnatal clinical outcome. Further evidences of partial or complete 12p tetrasomy in asymptomatic population are needed for a better evaluation of the associated risk.
CONFLICTS OF INTEREST
The authors declare no conflict of interests.