Sclerotic bone lesions in tuberous sclerosis complex: A genotype–phenotype study
Abstract
Tuberous sclerosis complex (TSC) is due to pathogenic variants in TSC1 or TSC2 genes resulting in hyperactivation of the mTOR pathway. Many organ systems can be affected, such as brain, skin, eye, heart, bone, kidney, or lung. Sclerotic bone lesions have been reported as frequent findings in TSC although they are not considered diagnostic criteria. The objective of this study is to characterize sclerotic bone lesions detected by chest CT in a large cohort of adult TSC patients and to correlate with genotype. Chest CT scans of 92 adult patients with a definite clinical diagnosis of TSC were reviewed. Sclerotic bone lesions were found in 82 cases (89%) and affected mainly the posterior vertebral elements. Patients without bone lesions had negative mutational studies of TSC1/TSC2 in 86%. Awareness of these lesions in TSC is important to avoid misdiagnosis with osteoblastic metastases.
1 INTRODUCTION
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder due to inactivating pathogenic variants in the TSC1 or TSC2 genes resulting in hyperactivation of the mTOR pathway (Crino, Nathanson, & Henske, 2006). Many tissues can be affected, such as skin, eye, heart, kidney, lung, or bone and most patients have involvement of the brain with tubers, white matter radial migration lines, and subependymal nodules. The presence of bone cysts had previously been considered a minor diagnostic criteria for TSC (Roach, Gomez, & Northrup, 1998), however, the new revised guidelines for diagnosis no longer consider them as diagnostic (Northrup & Krueger, 2013). Other bone lesions such as sclerotic bone lesions (SBLs) (Avila et al., 2010) and fibrous dysplasia (Gasparetto, de Carvalho Neto, Bruck, & Antoniuk, 2003; Li et al., 2015) have been reported in TSC. To the best of our knowledge, there is only one previous study characterizing bone lesions detected by chest computerized tomography (CT) in a large cohort of TSC patients (Avila et al., 2010). Our study is the first providing a genotype–phenotype correlation regarding sclerotic bone lesions.
2 MATERIALS AND METHODS
A retrospective review of the chest CT scans of 92 patients with a definite diagnosis of TSC, seen in our TSC clinic between January 2006 and June 2013, was performed. There were 30 males (33%) and 62 females (67%), with a mean age of 39.7 years (range: 18–81). Genetic testing (Sanger sequencing of TSC1 and TSC2 and study for TSC2 deletions) was available for 70 patients: 25 (35.7%) had pathogenic variants in TSC1, 29 (41.4%) in TSC2, and 16 (22.9%) had no mutation identified (NMI).
Bone lesions detected in these patients were characterized. In patients having more than one scan, the most recent study was reviewed. Axial CT reconstruction thickness was 1.25 mm in 28 patients, 2.5 mm in 61, and 5 mm in three patients. The following information was noted: age when the CT was performed, gender, mutational study, number, characteristics, and symptoms related to these bone lesions. Multiple lesions were defined as four or more lesions. The presence or absence of lymphangioleiomyomatosis (LAM) and multifocal micronodular pneumocyte hyperplasia (MMPH) was also noted.
The statistical analyses were performed using SPSS version 11.5 for Windows. An alpha level of 0.05 was used for all statistical calculations. Contingency tables were analyzed using Chi-square or Fisher exact test.
3 RESULTS
3.1 Patients with sclerotic bone lesions (SBLs)
Sclerotic bone lesions were detected in 82 patients (89%). There were 26 males (32%) and 56 females (68%), with a mean age of 39 years (range: 18–81). Of the 62 females total, 56 (90%) had SBLs, which was not significantly different from the frequency in males (26 males out of 30 had SBLs (87%). Mutational studies of TSC1/TSC2 had been determined in 63 patients: 25 patients (39.7%) had a pathogenic variant in TSC1, 28 (44.4%) in TSC2 and 10 patients (15.9%) had no mutation identified. None of the patients reported symptoms related to the SBLs. Sixty-one patients (74%; mean age: 36 [18–81]) had lung involvement. LAM and MMPH were present in 22/61 (36%; 91% females). Thirty-two patients had MMPH (52%; 53% females) and seven had LAM (12%; 100% females). The lung was normal in 21 patients (26%; 57% females; mean age: 41 [range: 24–69]).
3.2 Patients without sclerotic bone lesions
Ten patients (11%) did not show any bone lesions, including four males (40%) and six females (60%), with a mean age of 43 years (range: 19–64). Results from testing were available for seven patients: one patient had a pathogenic variant in TSC2 and six (86%) had no mutation identified (NMI). The patient with a TSC2 pathogenic variant was a 19-year-old female and was the youngest in this group. The predominant genotype in patients without bone lesions was no mutation identified (NMI) (86%). This difference was statistically significant (p < 0.001) compared to the group with bone lesions (patients with NMI: 15.9%). Five patients had lung involvement (50%; 100% females, mean age: 56 years [40–65]). Three had LAM and MMPH and two had LAM. The lung was normal in five patients (50%; 80% males, mean age: 31 years [range: 19–66]).
The presence or absence of SBLs in the 70 TSC patients with genetic TSC1/TSC2 studies are summarized in Table 1.
| SBLs | No SBLs | |
|---|---|---|
| Patients | 63 (90%) | 7 (10%) |
| TSC1 pathogenic variant | 25 (39.7%) | 0 |
| TSC2 pathogenic variant | 28 (44.4%) | 1 (14%) |
| No mutation identified (NMI) | 10 (15.9%) | 6 (86%) (p < 0.001) |
3.3 Characterization of bone lesions
A total of 77 of the 82 patients had four or more SBLs. Of those patients with less than four lesions, three were women and two men (mean age: 40 [range: 23–80]). Mutation analysis results were available for four: one had a pathogenic variant in TSC1, one in TSC2, and two had NMI. All lesions were hyperdense on CT. The distribution of lesions was: 82 patients (100%) had SBLs in the posterior elements of the vertebras (including lamina, pedicle, spinous, and transverse processes), 50 (61%) in the body, 59 (72%) in the ribs, 6 (7%) in the scapula, 1 in clavicle, and 1 in humerus (Figures 1-4).
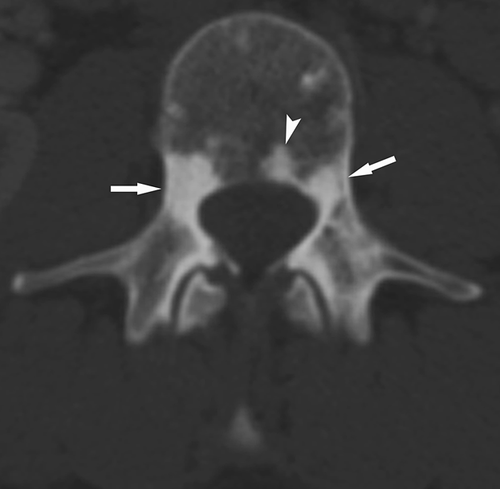

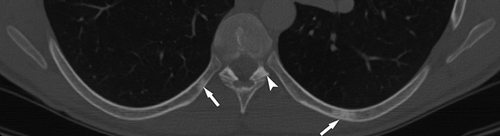
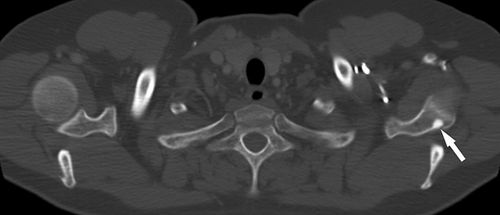
4 DISCUSSION
The previous consensus for clinical diagnostic criteria of TSC (Roach et al., 1998) included cystic bone lesions as a minor diagnostic feature of TSC; these are no longer included in the recent revised guidelines (Northrup & Krueger, 2013), as they were non-specific. Bone cysts are most commonly found in metacarpals and metatarsals, so radiographs of the extremities are required for their evaluation and these are not usually performed. They are typically cystic bone lesions with a sclerotic rim, and periosteal bone formation can be encountered as well (Evans & Curtis, 2000; Umeoka et al., 2008). Multiple foci of abnormal activity in Tc-99m HDP scintigraphy has also been described (Jonard, Lonneux, Boland, Malghem, & Jamar, 2001).
Sclerotic bone lesions of the axial skeleton are frequent manifestations related to TSC (Avila et al., 2010; Komar, Gabrielsen, & Holt, 1967) although they are not considered diagnostic criteria. They are best detected by chest CT (Avila et al., 2010), that is usually performed for assessing pulmonary manifestations, but may also be found in plain films (Komar et al., 1967), magnetic resonance (MR) imaging (Boronat, Barber, Pargaonkar, Chang, & Thiele, 2016; Stosic-Opincal et al., 2005), or bone scintigraphy (Song, Zhang, & Zhang, 2013).
Our study found a high frequency (89%) of these lesions in adults with TSC, supporting the results of another study in 107 adult TSC patients with chest CT that demonstrated a high prevalence of multiple SBLs (four or more) in 91% of cases, and 98% of patients showed at least one SBL (Avila et al., 2010). As already shown in that study, we also found that SBLs are preferentially located in the posterior elements of vertebrae, mainly pedicles and laminas. Our study is the first providing information about genotype in patients with these lesions. SBLs were found in TSC1 as well as TSC2 mutations. Interestingly, in the majority of patients without SBLs (86%) no mutation was identified. This finding correlates with a study showing a lower number of some TSC manifestations in patients with no mutation identified versus patients with TSC1 or TSC2 mutations (Camposano, Greenberg, Kwiatkowski, & Thiele, 2009).
There was no correlation of SBLs with LAM or MMPH lesions. In our cohort, gender and age distribution of LAM and MMPH was in accordance with previous literature, with far more penetrance in female gender and with age for LAM. On the other hand, SBLs affected males and females equally. As with other TSC lesions (Crino et al., 2006), the prevalence and expressivity of SBLs are expected to be higher with age. We did not perform a longitudinal study so we could not assess changes in number and size of these lesions with age. Also, as the youngest patients were 18 years old, we cannot rule out the possibility that the majority of these SBLs may have appeared during childhood or puberty. In fact, a recent abdominal MR longitudinal study of SBLs in children with TSC showed that 51 of 70 children (73%) had SBLs, and the youngest patient was 18 months old. New lesions appeared in 20 patients and growth of previous SBLs were documented in 14 patients (Boronat et al., 2016).
More awareness of SBLs is needed, as they may be easily and frequently detected by renal MR and chest CT performed as part of the clinical surveillance for TSC.
SBLs resemble foci of dense, compact bone within the medullary cavity of bones, which are called bone islands and are usually small and multiple (Komar et al., 1967). They are more prevalent in the spine and sacrum, with predilection to the vertebral bodies and posterior elements of the spine (Avila et al., 2010; Boronat et al., 2016; Umeoka et al., 2008). Histologically, they consist of thick mature bone trabeculae surrounded by normal spongiosa and have been classically considered hamartomatous lesions of no clinical importance (Holt & Dickerson, 1952). Since SBLs usually do not produce symptoms, patients do not undergo biopsy, and there are not molecular studies of any of these lesions, so it is not known if they follow a second-hit model, as already known for other TSC lesions (Crino et al., 2006).
The pathophysiology of SBLs is not known, but some data in the literature suggest that they may be of neural crest origin (Boronat, Shaaya, Doherty, Caruso, & Thiele, 2014). The neural crest is a multipotent cell population located between the neural and non-neural ectoderms. These cells migrate and colonize nearly all tissues of the embryo and give rise to a wide range of derivatives, such as melanocytes, neurons, and glia of the peripheral nervous system, and the majority of head and face tissues including meninges, intracranial vessels, bone, cartilage, and connective tissues (Dupin & Sommer, 2012; Nagoshi et al., 2008). Some TSC-associated lesions, such as hypomelanotic macules, lymphangioleiomyomatosis, renal angiomyolipomas, and cardiac rhabdomyomas react to the monoclonal antibody HMB-45, which is specific for fetal melanocytes (Kimura et al., 1997; Weeks, Chase, & Malott, 1994), indicating a neural crest origin of these lesions (Boronat et al., 2014; Delaney, Julian, & Stanford, 2014; Sarnat & Flores-Sarnat, 2005). In sporadic LAM, mutations in both alleles of the TSC2 gene have been detected in LAM cells but not in normal lung, kidney, or circulating lymphocytes (Carsillo, Astrinidis, & Henske, 2000) and these patients frequently also have extrathoracic angiomyolipomas (Maziak, Kesten, Rappaport, & Maurer, 1996) and SBLs (Avila et al., 2010). A chest CT study in sporadic LAM reported 30% of patients having between one and three SBLs and 3% with multiple lesions (Avila et al., 2010), suggesting a common neural crest origin for LAM, angiomyolipomas and SBLs (Boronat et al., 2014; Delaney et al., 2014).
There are two different pathways for the neural crest cells to reach axial and long bones, which are from mesodermal origin. One is that after populating the aorta-gonad-mesonephros, they move into the bloodstream and pass through the fetal liver to bone marrow. In mice they have been noted as a small population of self-renewing and pluripotent cells (Nagoshi et al., 2008). Another possible pathway for neural crest cells to reach the spine is by migrating through the sclerotomes, the structures that will form the vertebrae and ribs, on their way to form the sensory dorsal root ganglia and the sympathetic chain ganglia (Schoenwolf, Bleyl, Brauer, & Francis-West, 2008).
A recent study in mice with a neural crest-specific TSC1 deletion which showed sclerotic bone involvement has provided new information about the pathophysiology of bone involvement in TSC (Fang et al., 2015). Interestingly, in that study, early postnatal treatment with rapamycin, an mTORC1 inhibitor, completely rescued the aberrant bone mass, but late treatment could not. These data suggest that the upregulation of mTORC1 signaling in neural crest cells can expand the osteoprogenitor pool at an early postnatal stage by promoting proliferation, and this is responsible for the increased osteoblast number and higher bone mass acquisition at later postnatal stages. At 1 month of age in mice, the expanded osteoprogenitor pool has already been established and its maintenance does not require sustained mTORC1 activity. Thus, late rapamycin treatment could not prevent enhanced bone mass acquisition in these mice. This information suggests that a critical treatment window may need to be identified for the successful treatment of specific TSC manifestations.
An important clinical aspect of TSC SBLs is differentiating them from osteoblastic metastases. Awareness of SBLs in TSC is important for avoiding unnecessary clinical testing and misdiagnosis. Patients with TSC present frequently with tumors, and the finding of multiple sclerotic lesions in the spine may lead to the wrong diagnosis of osteoblastic metastases in some cases (Pui, Kong, & Choo, 1996), particularly if the clinical manifestations of TSC are mild or TSC has not been diagnosed yet. Moreover, the posterior aspects of the vertebral bodies, which also account for typical sites of spine metastases in adults (Stosic-Opincal et al., 2005), are the main location of SBLs in TSC. CT and MR imaging characteristics may help in differentiating these entities (Avila et al., 2010; Boronat et al., 2016; Rodallec et al., 2008). In two TSC cases with concomitant pulmonary tumors, bone scintigraphy was useful in distinguishing the TSC bone lesions from metastases (Pui et al., 1996; Song et al., 2013).
Limitations of our study are the retrospective nature and the lack of a control group. Also, as CT scans in our study were intended for pulmonary evaluation, they do not provide a characterization of sclerotic bone lesions through the body. However, the CT parameters used for chest imaging allowed a very good visualization of bone lesions.
5 CONCLUSION
SBLs are a frequent finding in TSC and are located mainly in the posterior aspects of the vertebrae. They are usually found incidentally in chest CT performed in TSC as surveillance for lung disease. Adult patients without SBLs on chest CT usually have negative TSC mutational studies. Awareness of these lesions in TSC may be important to avoid misdiagnosis as osteoblastic metastases.
Regarding their origin, some data support the neural crest as the origin of SBLs. Pathological and TSC mutational studies from SBLs tissue are needed to better understand the pathophysiology and molecular mechanisms leading to these lesions.