6q25.1 (TAB2) microdeletion syndrome: Congenital heart defects and cardiomyopathy
Abstract
Congenital heart defects (CHD) are the most frequent type of congenital anomaly and are often associated with genetic and chromosomal syndromes. Haploinsufficiency of TAB2 (TGF-beta activated kinase 1/MAP3K7 binding protein 2) has been proposed to cause valvular and cardiac outflow tract structural abnormalities. In this study, we describe 13 newly identified individuals with microdeletions of chromosome 6q25.1 that involve TAB2. One of the patients in our study cohort has the smallest deletion yet reported, affecting only TAB2. These were compared to 27 other patients reported in the published literature or DECIPHER to have similar microdeletions, for a total study group of 40 patients. Our study shows that individuals with TAB2 deletions are predisposed to developing a primary cardiomyopathy with reduced systolic function, even in the absence of CHD. Our study cohort also shares a number of non-cardiac phenotypic findings: characteristic dysmorphic facial features, intrauterine growth restriction and/or postnatal proportionate short stature, hypotonia, developmental delay and/or intellectual disability, and connective tissue abnormalities. We conclude that a microdeletion of 6q25.1 that includes TAB2 causes a distinctive, multi-systemic syndrome. The 6q25.1 microdeletion syndrome should be considered in a patient with cardiomyopathy or a CHD, especially valve and/or atrial or ventricular septal abnormalities, and with phenotypic features described in this study. We recommend that patients with a TAB2 deletion be screened longitudinally for systolic heart failure, even if an initial echocardiogram is normal.
1 INTRODUCTION
The prevalence of congenital heart defects (CHD) is nearly 1%, and an estimated 1.35 million infants are born with CHD each year worldwide (van der Linde et al., 2011). Over the last decade, with the advent of new genetic technologies—next generation sequencing, cytogenomic microarray analysis (CMA)—the discovery of genetic causes for CHD has accelerated. Copy number variants (CNVs) that result in altered gene dosage have been associated with a number of syndromes with cardiac malformations (Fahed, Gelb, Seidman, & Seidman, 2013).
Thienpont et al. (2010) identified a critical locus on chromosome 6q24-25 associated with CHD. They implicated TAB2 (TGF-beta activated kinase 1/MAP3K7 binding protein 2), which encodes TGF-beta-activated kinase one and MAP3K7-binding protein 2 (TAB-2), as the causal gene for structural CHD in this region. They postulated that TAB2 deletions cause valvular abnormalities and defects involving the left ventricular outflow tract (Thienpont et al., 2010).
We describe the cardiac findings and other clinical features of 13 previously unpublished individuals with a 6q25.1 microdeletion that includes TAB2, of whom seven are members of one four-generation family and six are unrelated individuals with de novo deletions. This large cohort of individuals with TAB2 deletions includes one whose deletion involves only the TAB2 gene. A novel observation in our cohort is the common occurrence of a primary cardiomyopathy with systolic heart failure, the severity of which cannot be fully explained by concurrent valvular and/or atrial or ventricular septal defects. Our findings suggest that TAB2 is not only important in cardiac development, but also plays an important mechanistic role in primary myocardial function.
Moreover, this cohort shares a number of non-cardiac findings: distinctive (dysmorphic) facial features, intrauterine and/or postnatal growth restriction with proportionate short stature, hypotonia, developmental delay and/or intellectual disability, and connective tissue abnormalities. These observations reinforce that a 6q25.1 microdeletion including TAB2 causes a multi-systemic syndrome.
2 MATERIALS AND METHODS
This study was approved by the Human Subjects Division and the Institutional Review Board (IRB) at the University of Washington Medical Center (UWMC). All the patients/legal guardians provided written informed consent to participate in this study, including review of relevant medical records.
2.1 Identification of patients with TAB2 deletion (Study group)
Ten individuals in four families with an isolated 6q25.1 microdeletion that included TAB2 were identified in the CMA Aberration Database of the Cytogenetics and Genomics Laboratory at UWMC (MyGCAD) and the Genoglyphix Chromosome Aberration Database (GCAD, PerkinElmer Inc.). Three other unrelated individuals were referred through a 6q deletion support group. For these 13 previously unpublished patients (Table 1), clinical notes from genetics and cardiology consultations, echocardiogram images and reports, and genetics test results were obtained and reviewed by a cardiologist, a medical geneticist, a laboratory geneticist, and a genetic counselor.
| Patient number | Coordinatesc [hg19] Chr6: | Chromosome band | Deletion (Mb) | Inherited? |
|---|---|---|---|---|
| P1 | 149622439–149745053 | 6q25.1 | 0.12 | Unknown |
| P2 | 149398652–149654593 | 6q25.1 | 0.26 | De novo |
| P4 to P9 | 148684028–150448233 | 6q24.3-q25.1 | 1.76 | Yes |
| P10 | 148684126–150469060 | 6q24.3-q25.1 | 1.78 | Unknown |
| P11 | 148607207–151405588 | 6q24.3-q25.1 | 2.80 | Unknown |
| P12 | 140073077–151572200 | 6q24.1-q25.1 | 11.50 | De novo |
| P13 | 141004908–152822158 | 6q24.1-q25.2 | 11.82 | De novo |
| Decipher 249394 | 148600786–150514126 | 6q24.3-q25.1 | 1.91 | Unknown |
| Decipher 250012 | 148795305–150738952 | 6q24.3-q25.1 | 1.94 | Yes |
| Decipher 261254 | 148736581–151187778 | 6q24.3-q25.1 | 2.45 | Yes |
| Decipher 265654 | 148937387–151939540 | 6q24.3-q25.1 | 3.00 | De novo |
| Decipher 281527d | 147163686–150184285 | 6q24.3-q25.1 | 3.02 | Yes |
| Decipher 250912 and 250911 | 146313209–149751890 | 6q24.3-q25.1 | 3.44 | Yes |
| Decipher 249489 | 146023678–149632217 | 6q24.3-q25.1 | 3.61 | Unknown |
| Decipher 279238 | 147241241–151702982 | 6q24.3-q25.1 | 4.46 | Unknown |
| Decipher 316709 | 145664452–155966517 | 6q24.3-q25.3 | 10.30 | De novo |
| Decipher 253173 | 144769787–156585724 | 6q24.2-q25.3 | 11.82 | De novo |
| Thienpont et al. (2010) Family N (II.3, III.1, III.2) | Breakpoint mapped to 149636547–149653526 | 6q25.1 | TAB2 disrupted by translocation | Yes |
| Weiss et al. (2015) | 149580983–149861981 | 6q25.1 | 0.28 | Yes |
| Salpietro et al. (2015) (Decipher 259674) | 149396427–150024732 | 6q25.1 | 0.63 | De novo |
| Thienpont et al. (2010) Family F (Patients Fp and Fm) | 148798307–150738307 minimum | 6q24.3-q25.1 | 1.94 | Yes |
| Caselli et al. (2007) (Decipher 1625; Thienpont et al. [2010] Patient D) | 148743936–151197299 | 6q24.3-q25.1 | 2.45 | De novo |
| Breckpot et al. (2010) Patient 7 (Thienpont et al. [2010] Patient A) | 143810202–150093227 minimum | 6q24.2-q25.1 | 6.28 | De novo |
| Bisgaard et al. (2006) Patient 1d (Thienpont et al. [2010] Patient E) | 149051973–156211229 | 6q25.1-q25.3 | 7.16 | De novo |
| Meloni et al. (2014) Patient 1 and 2 (Decipher 273678) | 144444361–152966111 | 6q24.2-q25.2 | 8.52 | Yes |
| Nowaczyk et al. (2008) Patient 2 (Thienpont et al. [2010] Patient C) | 146138307–156508308 minimum | 6q24.3-q25.3 | 10.37 | De novo |
| Nowaczyk et al. (2008) Patient 1 (Thienpont et al. [2010] Patient B) | 142308307–152438307 minimum | 6q24.1-q25.1 | 10.13 | De novo |
- a Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources http://decipher.sanger.ac.uk.
- b Unless otherwise noted, the 6q25.1 deletion was the only copy number variant detected in the patient.
- c When necessary, coordinates were converted to hg19 using the UCSC Genome Browser LiftOver tool.
- d Patient also has 89 kb deletion of [hg19]chr6:150435942-150524918 encompassing a portion of PPP1R14C.
- ePatient also has an apparently balanced paracentric inversion of chromosome 4 inherited from the father.
Eleven additional patients with TAB2 deletions contained in the Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources (DECIPHER) were also included (nos. 249394, 250012, 261254, 265654, 281527, 250911, 250912, 249489, 279238, 316709, 253173) (Table 1). A full list of centers who contributed to the generation of the data is available from http://decipher.sanger.ac.uk and via email from [email protected]. Funding for DECIPHER is provided by the Wellcome Trust (Firth et al., 2009). A review of the published literature yielded 16 other individuals with 6q25.1 microdeletions (Bisgaard et al., 2006; Breckpot et al., 2010; Caselli et al., 2007; Meloni et al., 2014; Nowaczyk et al., 2008; Salpietro et al., 2015; Thienpont et al., 2010; Weiss, Applegate, Wang, & Batista, 2015).
2.2 Identification of a comparison group
The comparison group included patients ascertained from MyGCAD and GCAD, who had CHD and/or cardiomyopathy listed in the indication for testing but did not have a microdeletion of 6q25.1 containing TAB2. A second comparison group, which had undergone CMA and consisted of 2,026 disease-free children (age 0–18 years) and the parents of these children (Shaikh et al., 2009), was used as the control group in the statistical analysis.
2.3 Cytogenomic microarray analysis
CMA of the patients (P) and parental follow-up testing was typically performed as part of clinical care, in various clinical laboratories. Thus, a variety of CMA platforms were used for testing patients in this cohort, including Agilent GGXChip + SNP v1.0 4 × 180 K, SignatureChipOS™, Agilent aCGH 60 K targeted array, Affymetrix Whole Genome-Human SNP Array 6.0, and Affymetrix Cytoscan HD array (Supplemental Table S1). Note that P3 was deceased prior to this study, and no materials were available for CMA. All genomic coordinates for 6q25.1 deletions are based on NCBI Build 37 (hg19) (February 2009).
CMA of P4–P9 and parental testing of P10 and P11 were done in the UWMC Cytogenetics and Genomics Laboratory using the Agilent GGXChip + SNP v1.0 4 × 180 K array platform. Purified genomic DNA and a normal control DNA were digested with restriction enzymes, labeled separately with contrasting fluorescence, and competitively hybridized to the microarray as specified by the manufacturer (Agilent Technologies, Santa Clara, CA). Arrays were scanned using a DNA Microarray Scanner with SureScan High-Resolution technology (Agilent Technologies). Whole genome microarray data were analysed using Agilent CytoGenomics 2.5 to identify CNVs. The global ADM2 algorithm with a threshold 6.0 and aberration filter for a minimum of five probes per region were applied. Log2R ratios provide information regarding copy number. CNVs were analyzed and interpreted using Genoglyphix software (PerkinElmer, Waltham, MA).
2.4 Statistical analysis
Statistical comparison using Fisher's exact test was performed to determine the p-value of the presence of 6q25.1 microdeletions containing TAB2 in individuals with a congenital heart defects versus that in a general population of disease-free individuals (Shaikh et al., 2009). Scores exceeding a p-value of 0.05 were considered significant.
3 RESULTS
3.1 CMA results
CMA detected isolated microdeletions of 6q25 containing TAB2 in 12 individuals from seven families (Table 1). P3 died before CMA could be done but, by pedigree analysis, most likely carried the deletion. No other CNVs of pathogenic or of uncertain clinical significance were detected. The deletions were non-recurrent, each with unique breakpoints (Figure 1, Table 1, Supplemental Figure S1). These deletions ranged in size from 123 kb (P1) to 11.5 Mb (P13) and contained one (TAB2) to 63 genes (Supplemental Table S1). The 123 kb deletion found in P1, containing multiple exons of TAB2 and all of SUMO4 (small ubiquitin-like modifier 4, a gene located in the last intron of TAB2), represents the smallest microdeletion of 6q25.1 involving TAB2 reported to date. The 256 kb microdeletion of 6q25.1 found in P2 only contains the 5′ end of TAB2.
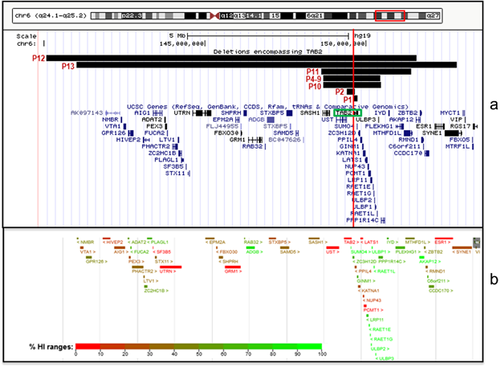
Eleven unpublished DECIPHER patients and 16 published patients with microdeletions that include TAB2 were also identified, with deletion size ranging from 280 kb to approximately 12 Mb (Table 1). In all but one of the DECIPHER patients, the deletion encompassing TAB2 was the only CNV submitted to DECIPHER, and in all but one of the published patients, the 6q25.1 deletion was the only genetic abnormality noted. Deletion size, genomic coordinates, and inheritance information for all known patients with TAB2 deletions are summarized in Table 1.
3.2 Statistical analysis
Among 425 individuals ascertained from MyGCAD and GCAD, who underwent CMA with CHD and/or cardiomyopathy listed in the indication for testing, an isolated microdeletion of 6q25.1 containing TAB2 was detected in 10 individuals from 4 families (2.35%), compared with 2 individuals in a general population of 2,026 disease-free individuals (0.098%) (Shaikh et al., 2009). The two-tailed p value calculated using Fisher's exact test was statistically significant at 0.0023, suggesting that deletions encompassing TAB2 are significantly enriched amongst individuals with CHD and/or cardiomyopathy.
3.3 Clinical findings
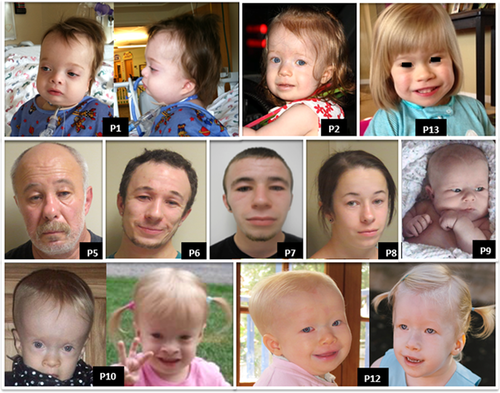
When our cohortʼs clinical features were compared to those of previously reported patients, we found that the frequencies of various features were similar (summarized in Table 2). A distinct pattern also emerged (summarized in Table 3). Individuals with 6q25.1 microdeletions containing TAB2 have multiple shared clinical features: CHD and/or cardiomyopathy; characteristic facial features including frontal bossing, short and/or narrow palpebral fissures, and retrognathia (Figure 2); intrauterine growth restriction (IUGR) and/or postnatal proportionate short stature (<3rd percentile for age); hypotonia; connective tissue abnormalities such as generalized joint laxity or hypermobility; and developmental delay and/or intellectual disability. Details of each of our 13 patients’ clinical presentation can be found in Supplemental Material SI.
| Patient number | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenotype | P1 | P2 | P5e | P10 | P11 | P12 | P13 | Number affected-current study (%)a | Number affected - previously reported (%)a, b |
| Gender | M | F | M | F | F | F | F | ||
| Age at last evaluation | 4 y | 2.5 y | 51 y | 4 y | 3 y | 3 y | 4 y | ||
| Cardiomyopathy/reduced ventricular systolic function | + | + | + | + | + | + | + | 7/7 (100%) | 1/1 (100%) |
| Congenital heart defect (CHD) | 5/7 (71%) | 12/13 (92%) | |||||||
| Aortic dilation | − | + | + | − | − | − | − | ||
| Aortic stenosis | − | − | + | − | − | − | − | ||
| Atrial septal defect | − | + | − | + | + | + | − | ||
| Bicuspid aortic valve | − | + | + | − | − | − | − | ||
| Myxomatousc mitral valve | − | − | + | − | − | + | − | ||
| Myxomatousc tricuspid valve | − | − | + | + | − | + | − | ||
| Overriding aorta | − | − | − | − | + | − | − | ||
| Patent ductus arteriosus | − | + | − | + | − | − | − | ||
| Pulmonic stenosis | − | − | − | − | + | − | − | ||
| Ventricular septal defect | − | − | − | − | + | − | − | ||
| Growth retardation | 6/7 (86%) | 16/16 (100%) | |||||||
| Intrauterine growth restriction | − | − | ND | − | + | + | + | 3/6 (50%) | |
| Proportionate short statured | + | − | + | + | − | + | + | 5/7 (71%) | |
| Hypotonia | − | + | ND | + | + | + | + | 5/6 (83%) | 4/5 (80%) |
| Developmental delay and/or intellectual disability | ND | + | − | + | + | + | + | 5/6 (83%) | 11/11 (100%) |
| Distinctive craniofacial features | 7/7 (100%) | 14/15 (93%) | |||||||
| Frontal bossing | + | + | + | + | + | + | + | ||
| Short and/or narrow palpebral fissures | + | + | + | + | + | + | + | ||
| Retrognathia | + | + | + | + | + | + | + | ||
| Connective tissue abnormalities | + | + | ND | − | + | − | + | 4/6 (67%) | 6/6 (100%) |
- +, documented to be present; −, documented to be absent; F, female; M, male; ND, not determined; y, years.
- a Total number of probands with the characteristic out of those in whom the characteristic could be determined.
- b See Supplemental Table S2 for details.
- c Myxomatous refers to abnormal valve thickening, redundancy, and/or prolapse.
- d The mean adult height summarized from 12 individuals is <3rd percentile.
- e Proband of one 4-generation family.
| Frequency (%)b | Sample size | Feature |
|---|---|---|
| ∼85% | 17/20 | Congenital heart defect (CHD) |
| Septal defects | ||
| Valvular abnormalities | ||
| ∼100% | 8/8 | Cardiomyopathy |
| Reduced ventricular systolic function | ||
| May occur even in the absence of CHD | ||
| ∼96% | 22/23 | Growth abnormalities |
| Intrauterine growth restriction | ||
| Postnatal growth restriction with proportionate short staturea | ||
| ∼95% | 21/22 | Characteristic dysmorphic facial features |
| Frontal bossing | ||
| Short and/or narrow palpebral fissures | ||
| Retrognathia | ||
| ∼94% | 16/17 | Developmental delay and/or Intellectual disability |
| Gross motor delay secondary to hypotonia | ||
| Some degree of intellectual disability | ||
| ∼82% | 9/11 | Hypotonia |
| ∼83% | 10/12 | Connective tissue abnormalities |
| Joint laxity or hypermobility | ||
| Redundant nuchal skin | ||
| Redundant forearm skin folds |
- a The mean adult height summarized from 12 individuals is <3rd centile.
- b % of PROBANDS with a TAB2 deletion in whom the characteristic could be determined (Table 2, Supplemental Table S2).
As delineated in Table 2, the majority of our patients with a TAB2 deletion had a CHD, including atrial and/or ventricular septal defects and various valve anomalies. At least one cardiac valve was abnormal in 11 of our 13 patients, and seven patients had two or three valve abnormalities. No patient had involvement of all four valves. P11 has a more complex CHD, including a secundum atrial septal defect (ASD), overriding aorta, pulmonic stenosis, and a membranous ventricular septal defect (VSD). A few patients presented with aortic root dilation and a bicuspid aortic valve (P2 and P5). While CHD is prominent, all our probands were born with or developed a cardiomyopathy resulting in clinically significant ventricular dysfunction. The majority of the patients developed symptoms of heart failure early in life after stressors, such as infection. However, P5 did not have documented biventricular dysfunction until adulthood. While the majority of the patients’ systolic heart function improved with time and medical therapy, a few died from heart failure complications (P3, P13).
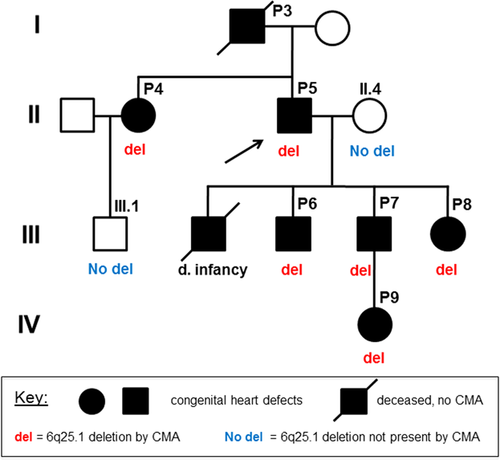
Patients P3–P9 in our cohort are members of a four-generation family with an inherited 6q25.1 microdeletion. This family has multiple cardiac valvular anomalies, and CMA testing on affected and unaffected members of the family showed that the microdeletion segregated with the cardiovascular phenotype (Figure 3). P3 and P5's eldest son were both deceased prior to this study, and no materials were available for genetic testing. P3 was assumed to have the deletion by pedigree analysis. P5's eldest son was born with hypoplastic left heart syndrome (HLHS) but was not included in this cohort, as it cannot be definitively determined whether his HLHS was related to the 6q deletion.
Despite having the identical 6q25.1 microdeletion, P3–P9 show some variation in their cardiovascular findings (Table 4). Common features include myxomatous (abnormally thick, redundant, and/or prolapsing) mitral or tricuspid valves and bicuspid aortic valve. Less frequent are pulmonic valve abnormalities, ASDs, and VSDs. Three of the seven family members have a known cardiomyopathy with systolic dysfunction, and P3 died from complications of heart failure.
| Affected members of 4-generation family | |||||||
|---|---|---|---|---|---|---|---|
| Phenotype | P3 | P4 | P5 | P6 | P7 | P8 | P9 |
| Gender | M | F | M | M | M | F | F |
| Age at last evaluation | 55 y | 56 y | 51 y | 22 y | 20 y | 19 y | 9 w |
| Cardiomyopathy/reduced ventricular systolic function | + | − | + | − | − | − | + |
| Congenital heart defect (CHD) | |||||||
| Aortic dilation | − | − | + | − | − | − | − |
| Aortic stenosis | − | − | + | − | − | − | − |
| Atrial septal defect | − | − | − | − | − | − | + |
| Bicuspid aortic valve | + | − | + | + | + | − | + |
| Myxomatousa mitral valve | + | + | + | + | + | + | − |
| Myxomatousa tricuspid valve | + | + | + | + | − | + | − |
| Pulmonic stenosis | − | + | − | − | − | − | − |
- +, documented to be present; −, documented to be absent; w, weeks; y, years.
- a Myxomatous refers to abnormal valve thickening, redundancy, and/or prolapse.
The most distinctive non-cardiac characteristics of the affected family members are their characteristic facial features (Figure 2) and short stature. The heights of the affected men range from 157 to 162 cm (5 feet 2 inches to 5 feet 4 inches). The affected women are 137 cm (4 feet 6 inches) and 142 cm (4 feet 8 inches) tall. They have no evidence of cognitive disability.
4 DISCUSSION
4.1 Clinical characteristics of the 6q25.1 microdeletion syndrome
Almost all individuals we identified with a 6q25.1 microdeletion that includes TAB2 have a cardiac abnormality. A CHD was found in 71% of patients newly reported in this study (Table 2), and a primary cardiomyopathy with reduced systolic function was present in 100% (Table 2), including some who had a structurally normal heart. The clinical presentations among individuals with these deletions differed, which could reflect the varying sizes of the alterations or sequence differences in other genes affected by the deletion. However, even among family members with the same deletion (P3–P9 in the current cohort), there were variable presentations of valvular heart disease and cardiovascular complications. In the one other published familial TAB2 microdeletion (Weiss et al., 2015), the cardiovascular manifestations also differed among family members. The most severely affected family member, the proband, has tetralogy of Fallot. The proband's mother has a bicuspid aortic valve, myxomatous/prolapsing mitral and tricuspid valves, and a paramembranous VSD. The maternal grandmother has myxomatous mitral and tricuspid valves, and her first pregnancy resulted in a male stillborn found to have a bicuspid aortic valve upon autopsy. A valve abnormality may not be recognized in infancy. P3 in our cohort presented with a heart murmur as a child, and II.3 from Weiss et al. (2015) was diagnosed with cardiac abnormalities at age 2. The two individuals (nsv522875 and nsv517889) with a TAB2 deletion in the pediatric control cohort (Shaikh et al., 2009) may also have been too young to have overt clinical manifestations at the time of ascertainment.
Other recognizable non-cardiac features of the 6q25.1 (TAB2) microdeletion syndrome include frontal bossing, short and/or narrow palpebral fissures, retrognathia, IUGR and/or short stature, hypotonia, connective tissue abnormalities (including joint laxity or hypermobility), and developmental delay and/or intellectual disability. Similarities to Noonan syndrome and Ehlers–Danlos syndrome were noted in some of our patients. More complete information on non-cardiac findings from additional individuals will be needed to best understand the complete phenotype associated with TAB2 deletions.
4.2 TAB2 as candidate gene for the 6q25.1 microdeletion syndrome
Haploinsufficiency or loss of function of TAB2 alone has been shown to be responsible for this multi-system disorder, such as in P2 in this cohort with only a TAB2 deletion (Table 1). Ackerman et al. (2016) recently reported a nonsense mutation in TAB2 (c.1491 T > A; p.Tyr497*) identified by whole exome sequencing in a 12-year-old boy with moderate cardiomegaly with right atrial enlargement, right ventricular hypertrophy, a secundum ASD, a paramembranous ASD, pulmonic stenosis, and thickened mitral, tricuspid, and aortic valves. He also has soft pale skin, hypotonia joint hypermobility, and mild dysmorphic facial features. Clinical exome analysis has identified TAB2 nonsense mutations in four other unrelated individuals whose phenotypic spectrum is similar to that seen in people with microdeletions encompassing TAB2, including structural cardiac abnormalities, cardiomyopathy, short stature, dysmorphic facial features, hypotonia, and connective tissue abnormalities (J. Rosenfeld, personal observation).
4.3 TAB2 haploinsufficiency and cardiovascular abnormalities
TAB2 is expressed in the endocardial cushion, which plays an important role in outflow tract and cardiac valve formation during embryonic development (Thienpont et al., 2010), and the structural alterations identified in individuals in this cohort and the other published studies are consistent with an effect in this tissue. From a population perspective, we found that 6q25.1 microdeletions affecting TAB2 are associated with CHDs (p = 0.0023). Among our patients, there was a wide range of cardiovascular findings, with significant intrafamilial phenotypic variability. Many individuals have outflow tract lesions that include bicuspid aortic valves, pulmonary valve dysplasia, ASDs, VSDs, aortic dilation, and mitral and tricuspid valve abnormalities. These lesions can be seen in individuals with connective tissue disorders such as Marfan syndrome or Loeys–Dietz syndrome (Kunkala et al., 2013). This suggests that the molecular mechanisms by which TAB2 deletions operate could share signaling pathways previously identified in those single gene disorders. For example, Loeys–Dietz syndrome is due to mutations in the transforming growth factor (TGF) beta receptors, disrupting TGF-beta signaling (Loeys et al., 2005). The protein encoded by TAB2 is also involved in TGF-beta signaling, downstream of the TGF-beta receptors (Ackerman et al., 2016).
The eldest child of P5, who had HLHS, died before the TAB2 deletion was identified in the family, and no tissue was available for genetic evaluation. However, multiple lines of evidence suggest that the cardiovascular phenotype caused by a TAB2 deletion may include complex CHD such as HLHS. Consistent with this idea, linkage analysis studies have identified a locus for HLHS on chromosome 6q (Hinton et al., 2009). Approximately 30% of fetuses with HLHS have associated genetic syndromes, and it is thought that 10–20% arise from CNVs (Benson, Martin, & Lo, 2016; Ciocca et al., 2015; Glidewell et al., 2015; Warburton et al., 2014). Families of individuals with HLHS are enriched with members who have bicuspid aortic valve, and the frequent occurrence of right and left-sided valve dysplasia seen in HLHS is consistent with the phenotype seen in TAB2 deletions (Hinton et al., 2007). More investigation is necessary to confirm that TAB2 haploinsufficiency is involved in this complex CHD, to identify other genes in addition to TAB2 that are dosage sensitive that may be accounting for the variable phenotype, particularly HLHS, and to determine whether these genes are involved in the same signaling pathway.
We were surprised to find that many individuals in our cohort had cardiomyopathy, dilated in some cases and non-dilated in others, with significant myocardial dysfunction that was not explained by their structural cardiac abnormalities. In our cohort, acute heart failure often coincided with an inflammatory event, which may have unmasked the underlying cardiomyopathy. Systolic heart failure occurred in both P2 and P13, neither of whom had significant CHD. The 6q25.1 microdeletion in P2 contains only TAB2, which is consistent with a model in which the gene not only plays a role in embryologic cardiovascular structural development, but also has a central role in cardiac myocyte function. While most of our patients had systolic heart failure early in life, some did not develop this complication until adulthood, as noted in P3 and P5.
TAB2 is known to be involved in immune and inflammatory cell-signaling pathways. It encodes TGF-beta-activated kinase 1 and MAP3K7-binding protein 2 (TAB-2), a protein that autophosphorylates and activates mitogen-activated protein kinase kinase kinase 7 (MAP3K7, also known as transforming growth factor-beta-activated kinase 1, or TAK1) (Xia et al., 2009). MAP3K7 is involved in innate immunity, and the inflammatory mediators involved in innate immunity are hypothesized to contribute to heart failure (Mann, 2015). Li et al. (2014) showed that MAP3K7 regulates myocardial homeostasis by inducing either pro-survival or cell death signaling within cardiac myocytes. Cell apoptosis and necrosis leading to loss of cardiac myocytes is a hallmark of cardiomyopathy, myocardial infarction, and end-stage heart disease. Cardiac-specific ablation of MAP3K7 in adult mice resulted in cardiac dysfunction, ventricular dilation, and cardiac hypertrophy. Cardiac tissue histology showed increased interstitial fibrosis and elevated markers of tissue necrosis. These mice were found to be more susceptible to heart failure when under pathologic cardiac stressors such as pressure overload or myocardial infarction. Li et al, (2014) proposed that with high MAP3K7 activity, tumor necrosis factor (TNF-a) activation induces formation of a complex involving MAP3K7 and TAB-2, leading to nuclear factor κB (NF-κB) signaling and pro-survival gene expression. When MAP3K7 activity is low, the pro-survival complex dissociates, and TNF-activation shunts preferentially toward a cellular necrosis pathway (Figure 4). They speculated that this process ultimately leads to myocardial dysfunction. When downstream regulators of the pro-necrotic pathway were inhibited, the cardiac phenotype of the Map3k7 knockdown mice was rescued (Li et al., 2014).
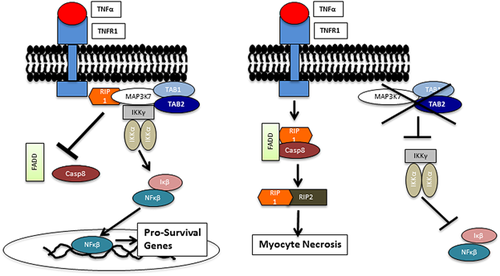
Li et al, (2014) findings provide a plausible explanation for the often stress-related systolic dysfunction in TAB2 haploinsufficient individuals (Figure 4). In the context of the patients described in this report, we hypothesize that decreased TAB2 expression reduces MAP3K7 activation, leading to increased myocardial necrosis under conditions of physiologic stress (e.g., pressure overload with aortic stenosis) or with an inflammatory process (e.g., systemic infection or respiratory illness). Direct studies involving TAB2 and myocardial function have not been reported, though a recent study (Ji et al., 2016) further implicates MAP3K7 and TAB-2 as important regulators in cardiac remodeling. Further investigation is warranted to better understand the role of TAB2 in heart failure.
5 CONCLUSION
We describe 13 newly identified individuals with overlapping 6q25.1 microdeletions encompassing TAB2, including one in whom only TAB2 is deleted. These individuals have a distinctive microdeletion syndrome, the characteristics of which include CHDs, risk of systolic heart failure even in the absence of a CHD, distinctive facial features, growth restriction, hypotonia, developmental delay and/or intellectual disability, and connective tissue abnormalities. The specific CHDs vary among our cohort, but most have valvular abnormalities and/or ASDs or VSDs. Bicuspid aortic valve is common, and at least one individual presented with concomitant aortic dilation requiring surgery. Many of our patients were born with or developed an idiopathic cardiomyopathy with at least transient myocardial dysfunction that was out of proportion to their cardiac valvular and atrial or ventricular septal defects. Our observations suggest that TAB2 plays a central role in cardiac myocyte homeostasis. Further investigation of TAB2 and related signaling pathways should lead to greater insights on mechanisms of heart failure.
Cytogenomic microarray analysis should be considered in patients with cardiomyopathy or a CHD, especially with multiple valve and/or ASDs or VSDs, with phenotypic features described in this study. We recommend prompt screening for systolic heart failure in all patients found to have a TAB2 deletion. The frequency of repeat echocardiograms should be based on clinical judgement and standard practice guidelines. Since systolic heart failure from a cardiomyopathy can manifest later in life, surveillance echocardiograms into adulthood may be indicated, even in the absence of significant CHD.
ACKNOWLEDGMENTS
We thank the patients and their families, without whom this work would not have been possible.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.