Diagnosis of CoPAN by whole exome sequencing: Waking up a sleeping tiger's eye
Abstract
Neurodegeneration with brain iron accumulation (NBIA) is a group of neurodegenerative disorders characterized by iron accumulation in the basal ganglia. Recently, mutations in CoA synthase (COASY) have been identified as a cause of a novel NBIA subtype (COASY Protein-Associated Neurodegeneration, CoPAN) in two patients with dystonic paraparesis, parkinsonian features, cognitive impairment, behavior abnormalities, and axonal neuropathy. COASY encodes an enzyme required for Coenzyme A (CoA) biosynthesis. Using whole exome sequencing (WES) we identified compound heterozygous COASY mutations in two siblings with intellectual disability, ataxic gait, progressive spasticity, and obsessive-compulsive behavior. The “eye-of-the tiger-sign,” a characteristic hypointense spot within the hyperintense globi pallidi on MRI found in the most common subtype of NBIA (Pantothenate Kinase-Associated Neurodegeneration, PKAN), was not present. Instead, bilateral hyperintensity and swelling of caudate nucleus, putamen, and thalamus were found. In addition, our patients showed a small corpus callosum and frontotemporal and parietal white matter changes, expanding the brain phenotype of patients with CoPAN. Metabolic investigations showed increased free carnitine and decreased acylcarnitines in the patientś dried blood samples. Carnitine palmitoyl transferase 1 (CPT1) deficiency was excluded by further enzymatic and metabolic investigations. As CoA and its derivate Acetyl-CoA play an essential role in fatty acid metabolism, we assume that abnormal acylcarnitine profiles are a result of the COASY mutations. This report not only illustrates that WES is a powerful tool to elucidate the etiology of rare genetic diseases, but also identifies unique neuroimaging and metabolic findings that may be key features for an early diagnosis of CoPAN.
1 INTRODUCTION
Neurodegeneration with brain iron accumulation (NBIA) is a group of inherited neurodegenerative disorders characterized by deposition of iron in the brain, mainly in basal ganglia. Clinical symptoms include progressive dystonia, spasticity, cognitive impairment, and neuropsychiatric abnormalities. Ten genetically defined NBIA subtypes are recognized. Eight are inherited as an autosomal recessive trait; the remaining two are of autosomal dominant or X-linked inheritance. The age of onset, clinical symptoms, and Magnetic Resonance Imaging (MRI) findings vary. The most common subtype (Pantothenate Kinase-Associated Neurodegeneration (PKAN, formerly known as Hallervorden-Spatz syndrome) is divided into a classic form with onset in early childhood and an atypical form with later onset. The classic form typically begins with cerebellar ataxia, followed by extrapyramidal symptoms, such as limb dystonia and cognitive decline. Other symptoms include pigmentary retinopathy and optic atrophy. PKAN has a characteristic brain MRI pattern, which consists of bilateral hyperintensity with surrounding hypointensity in the globus pallidus in T2-weighted images (“eye of the tiger sign”). PKAN is caused by mutations in PANK2, encoding the enzyme pantothenate kinase that catalyzes the first step of coenzyme A (CoA) biosynthesis. Identification of mutations in further genes led to recognition of a broader spectrum of NBIA phenotypes with different MRI abnormalities, including white matter changes, mild cortical atrophy, and brain stem and cerebellar atrophy. A summary of NBIA subtypes and their clinical and neuroradiologic features is given in Table 1.
| Name | Gene (inheritance) | Protein (function) | Subcellular localization | Neurological phenotype | Other features | Age of onset | Brain MRI: basalganglia | Other MRI features | References |
|---|---|---|---|---|---|---|---|---|---|
| PKAN | PANK2 (AR) | Pantothenate kinase 2 (catalyzes 1st step of CoA biosynthesis) | Mitochondria (intermembrane space) | Classic form: ataxia, dystonia, chorea, parkinsonism, cognitive decline, abnormal eye movements. Atypical form: speech defects, dystonia, tremor, psychiatric symptoms, cognitive impairment | Pigmentary retinopathy, optic atrophy (rare) | Classic form: early childhood. Atypical form: adulthood | T2-hypointensity with central hyperintensity of GP (eye-of-the-tiger-sign”), involvement of substantia nigra (pars reticularis) | ND, no white matter changes | Hortnagel, Prokisch, and Meitinger, (2003); Zhou et al. (2001) |
| PLAN | PLA2G6 (AR) | Phospholipase A2 G6 (catalyzes release of fatty acids from phospholipids) | Cytoplasma, plasma membrane, mitochondrial membranes, ER, nuclear envelope | Hypotonia, spasticity, bulbar dysfunction, cerebellar ataxia, abnormal eye movements, EEG-abnormalities, seizures in some patients | Optic atrophy | Early childhood to adulthood | T2-hypointensity of GP, but without central hyperintensity | Cerebellar atrophy, white matter changes | Arber et al. (2015); Liou et al. (2005); Morgan et al. (2006) |
| MPAN | C19orf12 (AR) | C19orf12 (unknown function) | Mitochondria (outer membrane) ER, mitochondria associated membrane | Speech and gait impairment, dystonia, parkinsonism, pyramidal- and extrapyramidal signs, psychiatric symptoms, axonal neuropathy | Optic atrophy | Childhood to adulthood | T2-hypointensities in GP and substantia nigra, central hyperintensity of GP (“eye of the tiger sign”) is rare | Cerebellar and cortical atrophy | Hartig et al. (2011); Venco et al. (2015) |
| BPAN | WDR45 (XL) | WDR45 (autophagy) | Phosphoinositol-3-phosphate-enriched membranes at the ER | Developmental delay, parkinsonism, dystonia, later dementia developing by early adulthood | ND | Childhood | T2-hypointensities in GP and substantia nigra with central hyperintense line | Cerebral and cerebellar atrophy | Haack et al. (2012) |
| FAHN | FA2H (AR) | Fatty acid 2-hydrolase (catalyzes hydroxylation of fatty acids, ceramide formation) | ER (membrane bound) | Gait disturbance, spastic paresis, ataxia, dystonia, loss of cognitive function, seizures (later) | Strabismus, nystagmus, optic atrophy | Childhood | T2-hypointensity of GP and substantia nigra | Pontocerebellar atrophy, subcortical and periventricular white matter changes, thin corpus callosum | Eckhardt, Yaghootfam, Fewou, Zoller, and Gieselmann, (2005); Kruer et al. (2012,2010) |
| Kufor-Rakeb syndrome | ATP13A2 (AR) | ATP13A2 (transmembrane transport of ions, regulation of intracellular cation homeostasis) | Lysosomes, other intracellular acidic vesicules, mitochondrial membrane | Parkinsonism, pyramidal tract signs, intellectual disability, dementia, supranuclear gaze palsy | ND | Adolesence | T2-hypointensity of GP, caudate, and putamen | Cerebral, cerebellar, and brain stem atrophy | Kruer et al. (2012); Ramirez et al. (2006); Ramonet et al. (2012); Schneider et al. (2010) |
| NFT | FTL (AD) | Ferritin light subunit (storage protein for cellular iron) | Cytosol, many intracellular compartments, extracellular space | Huntington disease like: Chorea, dystonia, parkinsonism, tremor, ataxia, mild cognitive decline | ND | Adolescence to older adulthood | T2-hypointensity in GP, putamen, caudate nucleus, thalamus, substantia nigra, and dentate. Later: cystic changes in basal ganglia | Cerebral and cerebellar atrophy | Curtis et al. (2001) |
| ACP | CP (AR) | Ceruloplasmin (copper transport protein and ferroxidase: oxidization of iron II–III) | Extracellular space, lysosome | Orofacial dystonia, dysarthria, chorea, tremor, gait ataxia, dementia | Retinal degeneration, diabetes, liver involvement (visceral iron deposition) | Adulthood | T2-hypointensity in GP striatum, thalamus, red nucleus, dentate nucleus | Cerebral and cerebellar Atrophy (mild), white matter changes | Kruer et al. (2012); McNeill, Pandolfo, Kuhn, Shang, and Miyajima, (2008); Musci, Polticelli, and Bonaccorsi di Patti, (2014); Schroder et al., 2007), |
| Woodhouse-Sakati syndrome | DCAF17 (AR) | DCAF17 (unknown function) | Nucleolus | Intellectual disability, dystonia, extrapyramidal symptoms, peripheral neuropathy (rare) | Hypogonadism, diabetes mellitus, alopecia, deafness, facial dysmorphism | Adolescence to adulthood | T2-hypointensity in GP | White matter changes | Alazami et al. (2008); Curtis et al. (2001) |
| CoPAN | COASY (AR) | Coenzyme A synthase (catalyzes last two steps of CoA biosynthesis) | Mitochondria (outer membrane, matrix, probably anchored to inner mitochondrial membrane) | Spasticity, dystonia, dysarthria, parkinsonism, developmental delay, cognitive decline, axonal neuropathy | Microcephaly (mild) | Early childhood | T2-hyperintensity of putamen, caudate nucleus, thalamus. Normal GP in the beginning. Later T2-hypointensity with central hyperintensity of GP (“eye-of-the-tiger-sign” | White matter changes, thin corpus callosum | Dusi et al. (2014), this report |
- PKAN, pantothenate kinase-associated neurodegeneration; PLAN, PLA2G6-associated neurodegeneration; MPAN, mitochondrial membrane protein-associated neurodegeneration; BPAN, beta-propeller protein-associated neurodegeneration; FAHN, fatty acid hydroxylase-associated neurodegeneration; NFT, neuroferritinopathy; ACP, aceruloplasminemia; CoPAN, COASY protein-associated neurodegeneration; ER, endoplasmatic reticulum; ERG, electroretinogram; GP, globus pallidus; ND, not described.
The exact mechanism leading to neurodegeneration and iron accumulation in NBIA is unknown. It is unclear if pathological accumulation of brain iron leads to neurodegeneration, or if deposition of iron is a secondary event following cell death. Only two forms of NBIA, neuroferritinopathy and aceruloplasminemia, are caused by mutations in genes for proteins directly involved in iron metabolism. The other genes are involved in diverse cellular pathways, such as lipid metabolism and CoA synthesis (Table 1).
Recently, mutations in the gene CoA synthase (COASY) have been identified as causative for a rare subtype of NBIA, COASY Protein-Associated Neurodegeneration (CoPAN) in Italian siblings (Dusi et al., 2014). In 2016, a third Italian patient with CoPAN has been described (Annesi et al., 2016). These three patients showed gait difficulties from early childhood, followed by oro-mandibular dystonia, dysarthria, spastic-dystonic paraparesis, parkinsonism, cognitive impairment, obsessive compulsive behavior, and axonal neuropathy. Eye involvement and white matter changes have not been described (Annesi et al., 2016; Dusi et al., 2014).
Here, we report on two siblings with CoPAN diagnosed by whole exome sequencing (WES), adding to the knowledge on the phenotype associated with loss-of-function mutations in COASY. Based on our findings and the previous report of Dusi et al. (2014), there is evidence that basal ganglia of patients with early CoPAN disease show a specific MRI pattern which can be used to distinguish CoPAN from other NBIA disorders.
2 MATERIALS AND METHODS
2.1 Patient ascertainment
Patients were recruited at the University Hospital Heidelberg, Heidelberg, Germany. The study was approved by the local ethics committee. Informed consent was obtained from both parents.
2.2 Genetic studies
Genomic DNA was isolated from both patients, their healthy sister, and parents from peripheral blood as described (Miller, Dykes, & Polesky, 1988). WES and data analysis was performed as previously described using the Heidelberg exome data analysis bioinformatics pipeline with the modification that the local control In-house database now included ∼300 exomes (Granzow et al., 2015). Variants with greater than 1% Minor Allele Frequency (MAF) in the Exome Aggregation Consortium (ExAC) database were considered as common alleles and removed from the candidate list. Using the ANNOVAR annotations (Wang, Li, & Hakonarson, 2010), coding variants were defined as missense or nonsense mutations, indels overlapping exonic regions, and variants ±2 base around the intron-exon junction (splice sites).
To confirm WES data by Sanger sequencing, exons 3 and 10 and adjacent intron boundaries of COASY (RefSeq NM_001042532.3, ensemble transcript ENST00000393818.2) were sequenced using Big Dye Terminator V1.1 cycle sequencing kit and ABI 3130xl genetic analyzer. Primer sequences and PCR conditions are available upon request.
3 RESULTS
3.1 Clinical report
3.1.1 Patient 1
The girl was the second child of non-consanguineous healthy parents from Turkey. She was born after 41 weeks gestation with a birth weight of 3470 g (41st centile), length of 50 cm (13th centile) and head circumference (OFC) of 32 cm (1st centile). Her psychomotor development was delayed. She started crawling at age 19 months, raised herself to standing position at age 22 months, was able to walk while holding on at age 23 months, and walked without support at 26 months. She spoke her first words at age 24 months. Neuropediatric examination at age 24 months showed hypotonia, broad based, ataxic gait, and ataxic hand movements. Evaluation using Griffith́s developmental scales at age 30 months demonstrated a developmental age of 11 months, indicating a severe developmental delay. At age 4 years 2 months exaggerated reflexes and mild pyramidal tract signs were seen. Behavior abnormalities with obsessive-compulsive symptoms and self-injurious behavior were obvious. On neuropediatric follow up examinations, persistent motor impairment and little progress of speech development, and perception were observed. Nerve conduction studies were not performed. The head circumference remained below the 3rd centile and the girl developed short stature. On last examination at age 8 years and 5 months her height was 112.2 cm (−4.2 SD), her weight 23.1 kg (10th centile) and her OFC 49.2 cm (−3.2 SD). Funduscopic examinations at ages 5 and 7 years were normal.
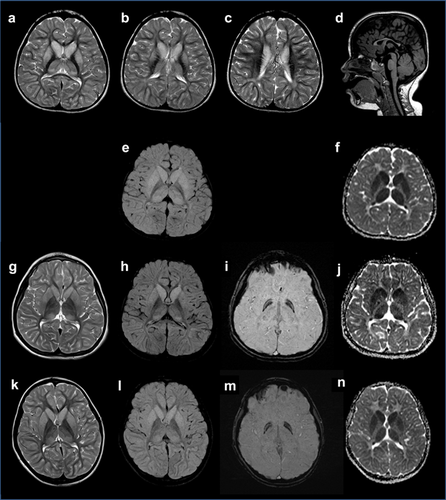
MRI examinations at 2 years and 11 months (Figure 1a–f) as well as at 3 years and 3 months demonstrated bilateral T2 hyperintensity and swelling of caudate nucleus, putamen, and thalamus. Diffusion-weighted (DW) MR imaging and apparent diffusion coefficient (ADC) indicated restricted diffusion. Globi pallidi were not affected. The anterior limb of the internal capsule showed regressive bilateral T2 and DW hyperintensity. In addition, the corpus callosum was small and frontotemporal and parietal white matter changes were observed. Cerebellum was normal. Magnetic resonance spectroscopy (MRS) gave normal results with no lactate peak seen. In follow-up MRI at age 8 years and 5 months (Figure 1g–j) the bilateral T2-hyperintensity and swelling of caudate nucleus, putamen, and thalamus was slightly regressive with still reduced apparent diffusion coefficient (ADC). MRS was normal.
3.1.2 Patient 2
The younger brother of patient 1 was born after 40 weeks of pregnancy with normal birth parameters (weight: 3500 g, 38th centile; length: 52 cm, 39th centile; OFC: 33.5 cm, 5th centile). His psychomotor development was delayed. He was able to sit without support at age 11 months, and could raise himself to a standing position at age 14 months. He spoke his first words at age 15 months. At age 20 months he could walk while holding on parent's hands, but showed a broad based, ataxic gait. At age 21 months he was able to walk without support. From 3 years on, progressive motor impairment and signs of spasticity were noticed. During the most recent neuropediatric examination at age 6 years he presented with hypotonia of the trunk, hyperreflexia, clonus of the Achilles tendon, and severe global developmental delay. The patient developed mild microcephaly and short stature. At age 7 years his height was 110.8 cm (−3 SD), weight 18.8 kg (−1.9 SD), and OFC 50.6 cm (−1.9 SD). Ophthalmologic assessment at age 2 years showed bilateral severe hyperopia (+5.5 diopters). Funduscopic examination at ages 2, 5, and 6 years were normal.
Brain MRI performed at age 7 years (Figure 1k–n) demonstrated bilateral T2- and FLAIR hyperintensity of nucleus caudatus, putamen, thalamus, and cortex with corresponding reduced ADC map, but without involvement of the globus pallidus. MRS was without specific changes.
3.2 Metabolic investigations
In patient 1 newborn screening was performed twice on two dried blood spot samples and showed increased free carnitine (CO), low levels of the long-chain acylcarnitines myristoylcarnitine (C16) and stearoylcarnitine (C18), and increased ratio of free carnitine to long-chain acylcarnitines (CO/(C16 + C18). Further measurements in dried blood samples confirmed the abnormal values, which were indicative of carnitine palmitoyl transferase 1 (CPT1) deficiency, a disorder of mitochondrial fatty acid oxidation. However, levels of CO, C16, C18, and ratio of CO/(C16 + C18) in blood serum were normal. Assay of CPT1 enzyme activity on cultured skin fibroblasts showed normal enzyme activity. Palmitate and oleate were oxidized normally in the patient's fibroblasts. Furthermore, rate of acetylcarnitine production from [13C]-palmitate was normal. Summing up, the results suggested no defect in mitochondrial fatty acid oxidation, at least in fibroblasts. Carnitine palmitoyl transferase 1 (CPT1) deficiency could be excluded. However, abnormal values for free carnitine (CO) and long-chain acylcarnitines (C16 and C18) in dried blood samples persisted during childhood (Supplementary Table S1).
In patient 2 newborn screening also showed elevated levels of CO, decreased levels of C16 and C18, and increased ratio of free carnitine to long-chain acylcarnitines (CO/(C16 + C18). Several further examinations of dried blood spots during childhood showed persistently increased free carnitine (CO) and elevated ratios of CO/(C16 + C18) (Supplementary Table S1). Levels of CO, C16, C18, and ratio of CO/(C16 + C18) in blood serum were normal at all times. No further investigations for CPT1 deficiency were initiated.
In both patients measurement of plasma amino acids and sterols, blood lactate and pyruvate, urine lactate and creatinine, urine organic acids, orotic acid, and screening for congenital disorders of glycosylation (CDG) by transferrin isoelectric focusing in serum gave normal results.
3.3 Whole exome sequencing
Greater than 98.79% of target regions on average had at least 10x coverage with a median-of-median base coverage of 94x. Exome sequencing variants were filtered by being consistent with an autosomal recessive disease model, their predicted damaging effect on protein function and their MAF. We considered homozygous or compound heterozygous non-synonymous single nucleotide variants (SNVs) and splice site variants (±2 base around the intron–exon junction) present in both affected children and with a MAF ≤ 1%. After applying these filter criteria the compound heterozygous missense variants c.C641T; p.(A214V) and c.C1495T; p.(R499C) in COASY and c.T1694C; p.(M565T) and c.C23T; p.(P8L) in KRI1 remained (for details see Supplementary Table S2). They were further assessed by their in silico predicted effect on protein function by seven different variant effect prediction tools (SIFT) (Ng & Henikoff, 2003), PolyPhen2 (Adzhubei et al., 2010), LRT (Chun & Fay, 2009), MutationTaster (Schwarz, Rodelsperger, Schuelke, & Seelow, 2010), MutationAssessor (Reva, Antipin, & Sander, 2011), FATHMM (Shihab et al., 2013), and PROVEAN (Choi, Sims, Murphy, Miller, & Chan, 2012) from dbNSFP v2.0 (Liu, Jian, & Boerwinkle, 2013). Subsequently, a manual literature search was performed for further information about gene function and to determine if the gene has been described in association with intellectual disability, neurological, or developmental disorders in humans. This narrowed the candidate list to the variants c.C641T; p.(A214V) and c.C1495T; p.(R499C) found in COASY (Coenzyme A synthase).
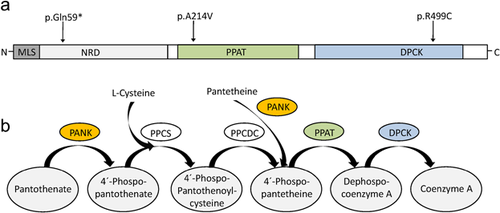
COASY encodes a bifunctional enzyme (CoA synthase) with phosphopantetheine adenylyltransferase (PPAT) and dephospho-CoA kinase (DPCK) activity, catalyzing the final two steps of Coenzyme A (CoA) synthesis (Daugherty et al., 2002). Recently, autosomal recessive mutations in this gene have been described as cause for a novel subtype of NBIA with early onset gait difficulties, spastic-dystonic paresis, and cognitive impairment (Dusi et al., 2014). The patients of Dusi et al. (2014) carried the same variant c.1495C > T; p.(R499C) as our patients, either homozygous (II–3, fam. 1) or compound heterozygous with a stop mutation (c.175C > T; p.(Q59*)) (II–2, fam. 2), respectively. The variant c.1495C > T; p.(R499C) affects a conserved amino acid in the DPCK domain, is predicted to be pathogenic by most prediction programs (Supplementary Table S2) and has been shown to abolish CoA biosynthesis due to reduced DPCK activity (Dusi et al., 2014). The other variant found in our patients, c.C641T; p.(A214V), is predicted to be damaging by most, but not all prediction programs (Supplementary Table S2). Figure 2b shows the domain structure of CoA synthase and the location of the variants. Both COASY variants of our patients were confirmed by Sanger sequencing, and both parents were shown to be heterozygous carriers (data not shown).
Other known genes for NBIA (as summarized in Table 1) including CPT1A were covered by the exome sequencing data but did not yield any putative pathogenic variant.
4 DISCUSSION
Using WES, we identified compound heterozygous variants in COASY [c.1495C > T; p.(R499C) and c.C641T; p(A214V)] in two siblings from a non-consanguineous Turkish family. The clinical phenotype of the patients is similar to that of three previously reported patients with COASY mutations, including ID, early onset ataxia, spastic paraparesis, pyramidal signs, and behavior abnormalities (Annesi et al., 2016; Dusi et al., 2014). Additional clinical features comprised mild microcephaly and short stature. Whereas the COASY variant c.1495C > T; p.(R499C) has been reported previously, the variant c.C641T; p.(A214V) has not been described. It affects a conserved amino acid in the phosphorpantetheine adenylyltransferase (PPAT) domain of CoA synthase. The presence of this variant in combination with the previously described pathogenic mutation c.1495C > T; p.(R499C) and the striking phenotypic similarities to the two previously reported patients strongly indicates that the diagnosis CoPAN can be made in these patients.
The patients highlight that the neuroimaging features of CoPAN early in disease may differ from other forms of NBIA. The “eye of the tiger sign” or other differences in the appearance of the globus pallidus, hallmark of most NBIA subtypes, was not present in the first MRIs of patient 1 at age 2 years 11 months, and 3 years 3 months, and was absent on the first brain MRI at age 5 years in one of the previously reported patients (pat. II–2, fam. 2 of [Dusi et al., 2014]). Instead, T2 hyperintensity and swelling of both caudate nuclei and putamina and mild hyperintensity in both thalami with normal globi pallidi was seen. Subtle changes in globus pallidi were evident for the first time at age 8 years, 5 months (patient 1), 7 years (patient 2) and 9 years (pat. II–2, fam 2 of [Dusi et al., 2014]), respectively. We suggest that the early neuroradiological changes of caudate nuclei, putamin, and thalami might be a CoPAN specific radiological marker that occurs before the typical increase of pallida iron content becomes evident. In addition, our patients showed a small corpus callosum and frontotemporal and parietal white matter changes, expanding the brain phenotype of patients with CoPAN.
It can be assumed that the COASY variants in our patients affect CoA biosynthesis and therefore the same cellular pathway as PANK2 mutations in PKAN (Figure 2b). For PKAN, it has been postulated that either the accumulation of the CoA precursor cysteine or the lack of CoA itself are responsible for the phenotype (for review see [Arber, Li, Houlden, & Wray, 2015]). Cysteine undergoes rapid auto-oxidation in the presence of iron, leading to free radicals. Cell death in the brain regions with high iron content, such as the globus pallidus (GP) and substantia nigra, might be the result (Hill & Switzer, 1984; Zhou et al., 2001). On the other hand, the lack of CoA and its derivates (e.g., acyl-Coa) might cause NBIA. Coenzyme A is an ubiquitous cofactor playing an important role in diverse metabolic pathways, including sterol and fatty acid synthesis, metabolism of amino acids, citric acid cycle, and β-oxidation. It has been hypothesized that reduced synthesis of fatty acids, cholesterol, and sphingolipids, which are essential for myelin production, leads to neurodegeneration and white matter changes in PKAN and CoPAN (for review see [Arber et al., 2015]). Consistent with this hypothesis decreased levels of sphingosine and the cholesterol precursors lanosterol and lathosterol were found in PKAN patients (Leoni et al., 2012). Kotzbauer, Truax, Trojanowski, and Lee, (2005) speculated that PANK2 mutations disrupt a mitochondrial-specific fatty acid synthase pathway essential for mitochondrial function. In addition, impaired metabolism of lipids and amino acids were suggested to result in insufficient mitochondrial energy production and cell death (Arber et al., 2015).
We preformed metabolic profiling to characterize the biochemical abnormalities in our patients. Although both variants were predicted to affect the brain specific isoform COASY beta as well as the ubiquitously expressed isoform alpha, most metabolic investigations gave normal results. Both patients showed normal sterols in plasma, including normal lanosterol, and lathosterol. Measurements of lactate, alanine, lactate, and lactate/pyruvat ration, markers for mitochondrial dysfunction, showed no abnormalities. In patient 1, mitochondrial fatty acid oxidation was normal, at least in fibroblasts. This is in contrast to previous observations in PKAN patients, which showed reduced lipid and cholesterol biosynthesis, impaired bile acid metabolism and elevated lactate suggestive of mitochondrial dysfunction (Leoni et al., 2012). Pank1−/− mice showed hypoglycemia and impaired fatty acid oxidation during fasting periods (Leonardi, Rehg, Rock, & Jackowski, 2010). Double knockouts of Pank1/Pank2 had defects in fatty acid oxidation, severe hypoglycemia, hyperketonemia, and died early (Garcia, Leonardi, Zhang, Rehg, & Jackowski, 2012). It is possible that the missense variants leave residual enzyme activity maintaining sufficient CoA levels at least in extra-cerebral tissues. Another explanation could be that—similar to the mouse model—metabolic abnormalities only occur during fasting periods and could be prevented by avoiding prolonged fasts.
Novel findings in our patients were the persistently increased levels of free carnitine (CO) and low levels of long-chain acylcarnitines (C16 and C18) in dried blood spots. This is a typical finding in patients with defect of CPT1, an enzyme that converts long-chain acyl-CoAs into acylcarnitines. Such a metabolic profile is extremely rare. Retrospective evaluation of more than 7000 patients attending the newborn screening at Heidelberg University Hospital showed that only one patient had the same abnormalities, he was affected by CPT1 deficiency. In patients 1 and 2, CPT1 deficiency was excluded, and the abnormal (acyl-) carnitine profile might be directly related to their CoPAN disease. The formation of long chain acyl-CoAs during fatty acid metabolism requires Coenzyme A. Impaired CoA production in CoPAN might lead to accumulation of carnitine in the cytosol, and reduced levels of long chain acylcarnitines (C16 and C18). Further studies are necessary to elucidate if the abnormalities of (acyl-) carnitine profile are restricted to our patients, or whether they occur in other CoPAN patients or may even be present as an early biomarker for the disease in patients with CoPAN and PKAN.
In summary, our report widens the phenotypic spectrum of COASY mutations and shows that unique basal ganglia MRI lesions and possibly characteristic metabolic findings are hallmarks of early CoPAN disease.