Whole exome sequencing of families with 1q21.1 microdeletion or microduplication
Abstract
Recurrent microduplications/microdeletions of 1q21.1 are characterized by variable phenotypes ranging from normal development to developmental delay (DD) and congenital anomalies. Their interpretation is challenging especially in families with affected and unaffected carriers. We used whole exome sequencing (WES) to look for sequence variants in two male probands with inherited 1q21.1 CNVs that could explain their more severe phenotypes. One proband had a 1q21.1 deletion transmitted from maternal grandmother, while the other had a paternal duplication. We found mutations in five genes (SMPD1, WNK3, NOS1, ATF6, and EFHC1) that could contribute to the more severe phenotype in the probands in comparison to their mildly affected or unaffected 1q21.1 CNV carrying relatives. Interestingly, all genes have roles in stress responses (oxidative/Endoplasmic Reticulum (ER)/osmotic). One of the variants was in an X-linked gene WNK3 and segregated with the developmental features and X inactivation pattern in the family with 1q21.1 deletion transmitted from maternal grandmother. In silico analysis of all rare deleterious variants in both probands identified enrichment in nervous system diseases, metabolic pathways, protein processing in the ER and protein export. Our studies suggest that rare deleterious variants outside of the 1q21.1 CNV, individually or as a pool, could contribute to phenotypic variability in carriers of this CNV. Rare deleterious variants in stress response genes are of interest and raise the possibility of susceptibility of carriers to variable environmental influences. Next generation sequencing of additional familial cases with 1q21.1 CNV could further help determine the possible causes of phenotypic variability in carriers of this CNV.
1 INTRODUCTION
Widespread use of chromosomal microarray analysis (CMA) has proven to be a valuable tool for detection of submicroscopic DNA copy number variants (CNVs) associated with intellectual disability (ID) and/or autism. It has led to the identification of novel microduplication/microdeletion syndromes (Torres, Barbosa, & Maciel, 2016) and CNVs predisposing to neurodevelopmental abnormalities. Deletions and duplications of the distal 1q21.1 chromosome region (1.35Mb, from 146533376 to 147883376, hg19) belong to this later group of “susceptibility” CNVs based on increased frequency in patients with DD (0.2% for duplications and 0.29% for deletions) relative to controls (0.03%) (p < 0.0001) (Rosenfeld, Coe, Eichler, Cuckle, & Shaffer, 2013). Carriers of 1q21.1 deletions and duplications have variable clinical features which include developmental delay (DD), language disorder, mild-to-moderate ID, autism spectrum disorders (ASDs), anxiety and mood disorders, mild and non-distinctive facial dysmorphism, congenital heart defects, seizures, abnormalities of the skeletal system, and short stature (OMIM #612474 and #612475). Microcephaly is typically associated with deletions and macrocephaly with duplications of this region. Milder or normal phenotypes tend to be observed in carrier parents and include a wide range of learning and behavioural difficulties (ADHD, anxiety/depression, and antisocial behaviours) (Brunetti-Pierri et al., 2008; Mefford et al., 2008). Despite the variable phenotypes, CNVs involving this region show little variation in size or breakpoints (Harvard et al., 2011). The commonly duplicated or deleted region in 1q21.1 contains eight OMIM genes (PRKAB2, FMO5, CHD1L, BCL9, ACP6, GJA5, GJA8, and GPR89B) which are involved in multiple pathways. Apparently identical CNVs in unaffected and affected family members are particularly hard to interpret and associate with the abnormal development.
Recently, exome sequencing has provided the opportunity to look for additional causes of phenotypic variability in carriers of familial CNVs. For example, the presence of Thromobocytopenia with Absent Radium (TAR) syndrome features in some carriers of the proximal 1q21.1 deletion (breakpoints 145386506-145748067bp, hg19), but not others, has been explained by the presence of sequence variants in RBM8A gene on the non-deleted copy of the 1q21.1 region only in the subjects with TAR syndrome (Albers et al., 2012). Similarly, exome sequencing unmasked a pathogenic variant in the SNAP29 gene on the non-deleted copy of 22q11.2 region in subjects with atypical DiGeorge syndrome and deletion of 22q11.2. (McDonald-McGinn et al., 2013) Finally, we and others used exome sequencing and identified rare deleterious variants outside of familial CNVs (e.g., 16p11.2 duplication) contributing to the phenotypic variability which could not be explained only by the CNVs (Classen et al., 2013; Dastan et al., 2016).
Whole exome sequencing (WES) to search for modifying mutations in carriers of 1q21.1 CNV has not yet been performed. In the present study, we assessed the presence of rare deleterious variants in the 1q21.1 region, and genome-wide, in two probands from two different families, carrying a deletion or a duplication of 1q21.1, respectively.
2 MATERIALS AND METHODS
2.1 Subjects
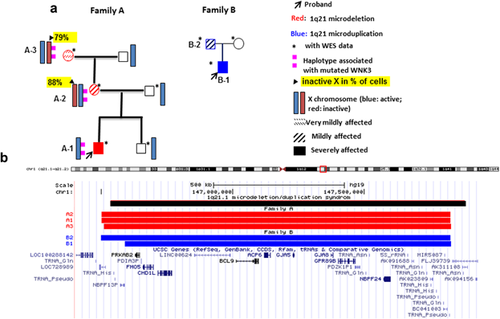
Two unrelated families carrying a 1q21.1 deletion or duplication (Family A and B, reported in our previous study (Harvard et al., 2011) as Family A and C, respectively) were included in this study (Figure 1). Their phenotypes and CNV analysis were summarized in Supplementary Table S1. The study was approved by the Committee for Ethical Review of Research involving Human Subjects, University of British Columbia. All subjects gave written informed consent for participation in our study.
2.2 Whole genome chromosome microarrays analysis (CMA)
The q21.1 CNVs were identified using the high resolution Affymetrix 2.7M SNP array platform according to the manufacturer's protocols (http://www.affymetrix.com) (Affymetrix Inc., Santa Clara, CA) and described in detail previously (Harvard et al., 2011; Qiao et al., 2012).
2.3 Whole exome sequencing (WES)
WES was performed on eight subjects from the two unrelated families (A and B) with 1q21 CNV(s) (Figure 1). In family A, this included three 1q21.1 deletion carriers (male proband-A1, mother-A2, and grandmother-A3) and the proband's unaffected non-carrier father and male sibling. In family B, two duplication carriers (male proband-B1 and father-B2) and the proband's non-CNV carrier, unaffected mother were sequenced. DNA extracted from peripheral blood was sent to BGI (BGI, China). Briefly, WES was performed with Agilent Sure Select exome capture kit version (50 MB) to generate libraries sequenced on an Illumina HiSeq 2000 platform. BAM files from all cases were combined into one .vcf file containing SNPs and inDels, using the pipeline provided by Seven Bridges (https://www.sbgenomics.com/). The .vcf files are imported into Golden Helix (GH) SNP and Variation Suite (SVS) v8.1.5 software (Golden Helix, Inc., Bozeman, MT, https://www.goldenhelix.com). Both single nucleotide variants (SNVs) and small insertions and deletions (Indels) were classified, annotated, and functionally profiled using multiple databases included in the software. Putatively pathogenic variants were evaluated manually based on multiple publicly available databases in combination with inheritance pattern (Qiao et al., 2016). Specifically, the filtering process included retention of variants that met the quality-control metrics that is, Read Depth ≥10, Genotype Quality ≥20, and Alternate allele read ratio ≥0.25. Rare variants with minor allele frequency (MAF) <1% in general population were selected based on Exome Variant Server (EVS) (NHLBI Exome Sequencing Project [ESP] [NHLBI ESP6500SI, Seattle, WA. http://evs.gs.washington.edu/EVS/]), 1000 Genomes project (http://www.1000genomes.org/data), and ExAc (http://exac.broadinstitute.org/). Variant impact on protein function was evaluated by dbNSFP NS Functional Predictions program (Liu, Jian, & Boerwinkle, 2013). In addition, we manually added Indel function prediction using Indel SIFT webtool (http://sift.bii.a-star.edu.sg/www/SIFT_indels2.html) and Indel conservation prediction using PhastCons46wayPlacental and PhyloP46wayPlacental from UCSC (https://genome.ucsc.edu/). In total, 10 prediction tools were used for evaluation of variant pathogenicity (SIFT Pred, Polyphen2 HDIV Pred, Polyphen2 HVAR Pred, LRT Pred, MutationTaster Pred, MutationAssessor Pred, FATHMM Pred, MetaSVM Pred, MetaLR Pred, and Indel SIFT) and three for conservation scores (GERP + + RS, PhastCons46wayPlacental, and PhyloP46wayPlacental). Golden Helix Genome Browser was used for checking data quality in comparison with our internal exome sequenced control cases. For selecting a pool of genes with rare deleterious variants in the probands for further enrichment analysis, we focused on 1) variants with MAF<1%, pathogenicity score ≥1, and conservation score ≥1 provided by SVS software; 2) all loss of function variants (e.g., splicing, stop gain, stop loss) inherited from the 1q21.1 CNV carrier parent or de novo; 3) missense variants with all types of heritance. For selecting individual mutations that may be modifiers of 1q21.1CNV, we screened the above pool of variants for those that 1) were de novo in the proband or inherited as homozygous, compound heterozygous or X linked (excluding heterozygous inherited missense variants); 2) with expression in brain based on Human Brain Transcriptome database (Gilissen et al., 2014). We also assessed the variants based on their role in ID (Gilissen et al., 2014) and used other databases (e.g., PubMed and MGI), for phenotype correlation.
2.4 Sanger sequencing
Sanger sequencing was used for confirmation of candidate variants from WES. Primers were designed using Primer Express 3.0. PCR was preformed according to the Invitrogen protocol for Taq DNA polymerase and cleaned products run on an ABI 3130xl sequencer. Primer sequences and Sanger profiles confirming selected deleterious variants are available on request.
2.5 Non-random X inactivation
Due to X-linked mutation in WNK3 in family A the proband's mother and grandmother with 1q21.1 deletion were tested for non-random X inactivation utilizing the HUMARA assay DNA extracted from whole blood as previously described (Allen, Zoghbi, Moseley, Rosenblatt, & Belmont, 1992). Additionally, seven microsatellite markers were genotyped (using standard procedures) along the short and long arms of the X and Y chromosome to try to establish the individual haplotypes of the family members (see Supplementary Figure S1 for details on markers).
2.6 RNA expression of genes with deleterious variants in patients’ cell lines
Confirmation of RNA expression for ATF6, NOS1, and EFHC1 was performed in Epstein-Barr Virus (EBV)-transformed lymphoblastoid cell lines from proband B1 and his carrier father B2 by reverse-transcription PCR using GeneAmp Gold RNA PCR Core Kit (Applied Biosystems, Melbourne, Australia) as previously described (Wen et al., 2013). Cell lines were not available for family A. Values were considered statistically significant with a p-value of <0.05. The primers were designed for exons 11–12 of ATF6: 5′-TTG CTT TAC ATT CCT CCA CCT-3′ (F); 5′-CTT GGT CCT TTC TAC TTC ATG TCT-3′ (R); exons 1–3 of ATF6: 5′-ACC ATG GAG TCA CCT TTT AGC-3′ (F); 5′-CAT ACG TCT CAT TTG CTG CTT C-3′ (R); exon 26–27 of NOS1: 5‘-CAAGAACAAGGGGGTCTTCA (F); 5‘- CAGGGCTCGGTACACAGACT-3‘ (R); and exon 8–9 ffor EFHC1: 5‘- GGTATCATTGGGGGCAAGTA-3‘(F); 5‘- AAGGATGATGAACCGGTGAC-3‘(R).
2.7 Western blot analysis
Western blotting was performed to detect ATF6α protein expression level in lymphoblastoid cell lines as previously described (Zhang, Lai, Teodoro, & Volchuk, 2009). Briefly, the transformed cell lines were lysed in lysis buffer (1% TX-100, 100 mM KCL, 20 mM HEPES, 2 mM EDTA, pH7.3, 1 mM PMSF, and Roche protease inhibitors), left on ice for 30 min, then centrifuged at 4 °C for 10 min. The supernatant was collected and the protein concentration was determined using a BCA protein assay kit. For the ATF6α Western blots 50 μg of protein was resolved using SDS-PAGE and immunoblotted using an antibody directed against ATF6α (mouse monoclonal 1–7 from BioAcademia Inc.). β-tubulin was used as a loading control (anti-β-tubulin, Abcam ab6046).
2.8 ER stress response analysis
The Endoplasmatic Reticulum (ER) stress response was monitored to examine the effect caused by ATF6 deleterious variant in proband B1 and his father B2 who carried the ATF6 variant and a 1q21.1 duplication. Thapsigargin and tunicamycin were used to induce ER stress. Thapsigargin causes altered ER calcium levels and tunicamycin inhibits protein glycosylation in the ER, which perturbs protein folding leading to misfolded protein accumulation in the ER. ATF6, a transcription factor, is activated under these conditions and is known to regulate several ER stress response genes including XBP1 and ER chaperones (So, Warsh, & Li, 2007). EBV-transformed lymphoblastoid cell lines derived from the proband and his father or control cell lines were treated or not with thapsigargin (1 μM for 16 hr) or tunicamycin (2 μg/ml for 16 hr). Total RNA was subsequently isolated, reverse transcribed and the cDNA was used for real-time PCR analysis using SYBR green qPCR or the Taqman Gene Expression System (Applied Biosystems) as described previously (Zhang et al., 2009). ATF6 target genes analyzed are: GRP94 (HSP90B1), BiP (HSPA5, GRP78), SDF2L1, DNAJC3, and XBP1. Primer information is available upon request.
2.9 In silico analysis
We used a public free webtool called WebStalts (http://www.webgestalt.org) for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and disease association enrichment analysis of genes with putatively pathogenic variants from the two probands of the families. Briefly, enrichment analysis was performed on pool of genes with all types of rare deleterious variants (frameshift, stopgain, stoploss, initiation codon, splice site, and missense) with pathogenicity score ≥1 and conservation score ≥1, MAF < 1% and with all inheritance patterns in the two probands (collectively 143 genes; 60 in proband from Family A and 83 in proband from Family B). Positive control genes included 307 genes shared between a list of known ID genes (∼700 genes) (Vissers, Gilissen, & Veltman, 2016) and a website containing known ID genes (∼350 genes) (http://gfuncpathdb.ucdenver.edu/iddrc/iddrc/data/iddrcgname.html). Negative control gene pool contains 149 genes randomly extracted from whole human genome by in silico method as described previously (Supplementary Table S2) (Qiao et al., 2010).
3 RESULTS
3.1 Clinical description
The detailed phenotypes of all 1q21.1 CNV-carriers from Family A and B are listed in Supplementary Table S1 and reported previously (Harvard et al., 2011). Briefly, the probands A1 (1q21.1 del) and B1 (1q21.1 dup) were more severely affected than their carrier parents and show microcephaly (A1), macrocephaly (B1), ID and other congenital abnormalities. Carrier parents had mild developmental/behavioural phenotypes, such as learning difficulties (A2 and B2), ADHD (B2) or memory loss (A2). A3 was considered cognitively and behaviourally normal. Facial dysmorphism was also more severe in probands A1 and B1 compared to parent-carriers. It was mild in A2 and non-existent in B2. All carriers of the 1q21.1 deletion from family A had short stature.
3.2 CNV analysis
The 1q21.1 CNV breakpoints are shown in Figure 1 and Supplementary Table S1. No secondary CNVs were detected in our cases that could be considered pathogenic and contributing to the phenotypes. The 1q21.1 CNV region ranges from 145257168 to 148545581 (hg19) with a median size of 1.3Mb. The overlapping genes included FMO5, CHD1L, LINC00624, BCL9, ACP6, GJA5, and GJA8. Little variation of CNV content was observed between family members with the same CNV (Figure 1).
3.3 Candidate variants from WES analysis
WES showed no rare deleterious variants in the 1q21.1 CNV region or the 10 Mb flanking region in any of the five subjects who carried 1q21.1 CNVs. In Family A, exome analysis revealed two rare, deleterious variants in the proband with 1q21.1 deletion: a compound heterozygous variant in SMPD1 (chr11: 6415245, c.1460C>T, p.Ala487Val and chr11: 6411836, c.8G>A, p.Arg3His) and a grand-maternally inherited missense variant in X-linked gene WNK3 (chrX: 54265343, c.3841C>T, p.Arg1281Trp) (Table 1). The X inactivation study and microsatellite analysis suggests that in the grandmother the X chromosome with WNK3 mutation (WNK3 v) is inactive in 79% of cells, while the majority (88%) of maternal cells had the mutation on the active X chromosome. The same chromosome with the WNK3 mutation was transmitted to proband A1 (Supplementary Figure S1), while his male sibling inherited the maternal X chromosome with the wild-type gene, WNK3 wt. The male sibling did not have the deleterious variants found in his affected brother (A1).
| Proband | Gene | Inheritance | Position | Protein | Classification | Gene function-general | Gene function-stress related |
|---|---|---|---|---|---|---|---|
| A1 in family A | SMPD1 | Compound heterozygous | 11:6411836-SNV/11:6415245-SNV | p.Arg3His/p.Ala487Val | Nonsynonymous SNV (both) | Sphingomyelin/ceramide signal transduction (Fernandez et al., 2013; PMID: 23707365) | ER stress (Fernandez et al., 2013; PMID: 23707365); oxidative stress (Li et al., 2012; PMID: 22890197) |
| WNK3 | X-linked hemizygous | X:54265343-SNV | p.Arg1281Trp | Nonsynonymous SNV | Electrolyte homeostasis (Kahle et al., 2008; PMID: 17961084); regulates the cation-chloride cotransporters (Begum et al., 2015; PMID: 26069258) | Osmotic stress (Kahle et al., 2008; PMID: 17961084) | |
| B1 in Family B | EFHC1 | De novo | 6:52303273-SNV | p.Arg153Trp | Nonsynonymous SNV | Calcium homeostasis, neuronal migration (de Nijs et al., 2012; PMID: 22926142) | Oxidative stress (Katano et al., 2012; PMID: 22226147) |
| NOS1 | Paternal | 12:117725997-SNV | p.0? | Initiation codon | Neurotransmission (Ziolo & Houser, 2014; PMID 24801117). | Oxidative stress (Drechsel et al., 2012; PMID: 22488161) | |
| ATF6 | Paternal | 1:161753884-Del | p.Pro118 fs | Frameshift deletion | Functions as an ER stress sensor/transducer (Yamamoto et al., 2007; PMID: 17765680). | ER stress (Sela et al., 2012; PMID 22577136) |
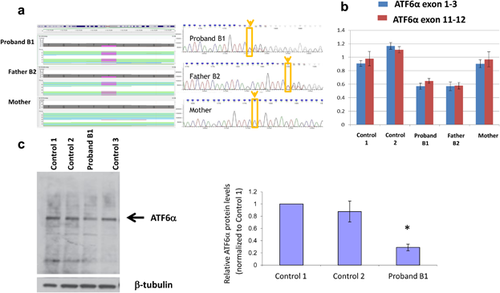
In Family B with the 1q21.1 duplication, a de novo missense mutation in EFHC1 (chr6: 52303273, c.457C>T, p.Arg153Trp), and two paternally inherited LOF mutations were identified (Table 1). The ATF6 mutation (chr1:161753884delC, c.352delC, p.Pro118 fs) (Figure 2a) is located within exon four of ATF6 and predicted to cause both a frameshift and disrupt a splice site. The other mutation affects the initiation codon of NOS1 (chr12:117725997, Init Codon, c.1A>G, p.0?). All variants were confirmed by Sanger sequencing.
3.4 Expression level detection and functional analysis of genes with rare deleterious variants
The RNA and protein expression of the genes with mutations were assessed in family B as transformed lymphoblastoid cell lines were available from B1 and B2. ATF6 showed decreased RNA and protein expression level in B1 and B2 who carried the heterozygous mutation compared to normal controls (Figure 2b,c). Expression of NOS1 and EFHC1 could not be detected in control lymphoblasts and was not pursued in patient cells. It was however detectable in control human brain (not shown).
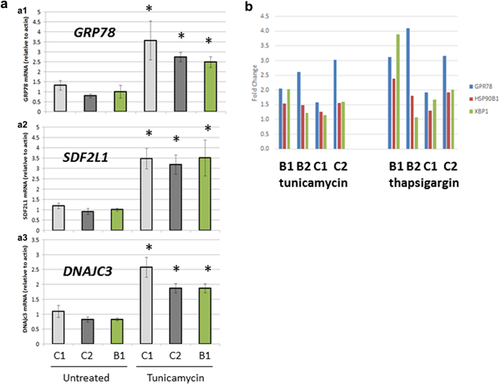
Because ATF6α expression was detectable but lower in B1 and B2 than controls, its activation in response to ER stress could be tested in lymphoblast cell lines. Thus, we explored if the ATF6α mutation and its corresponding reduction in mRNA and protein expression would affect the cells ability to respond to ER stress. We hypothesized that reduced ATF6α levels in the patient cell lines would result in a compromised ability to induce genes known to be regulated by ATF6α in response to ER stress. ER stress was induced by treating cells with tunicamycin or thapsigargin and ATF6α downstream target gene expression was compared in control cell lines and in cell lines from B1 and B2 with ATF6α mutations (So et al., 2007). As expected, thapsigargin and tunicamycin caused an induction of ATF6-regulated genes including GRP94 (HSP90B1), BiP (HSPA5, GRP78), SDF2L1, DNAJC3, and XBP1 in control and patient cell lines (Figure 3a). However, no significant difference in the fold induction of these genes was observed between the ATF6 mutation carriers and controls (Figure 3b).
3.5 Pathway analysis of candidate variants
In silico analysis using WebStalts showed that the pool of the genes with rare deleterious variants from the two probands is enriched in metabolic pathways, arginine and proline metabolism, sphingolipid metabolism protein processing in ER and protein export (adjusted p value < 0.01) (Table 2). Metabolic pathways were the top enriched pathways in both probands and for positive control ID genes but not enriched for negative control gene pool obtained randomly from the genome. Similarly, Nervous System Diseases and Mental Disorders are the top enriched diseases associated with the mutated genes in the probands, while mental retardation is the top disease associated with positive control genes known to be mutated in patients with ID. When the enrichment analysis of all genes with rare deleterious variants from one proband was performed independently from the genes from the other proband comparable results were obtained that is, two pathways (Metabolic pathways and Protein processing in ER) and two disease categories (Nervous System Diseases and Central Nervous System Diseases) were enriched.
| From 2 probandsa | Positive control genesb | Negative control genesc | |
|---|---|---|---|
| No. of genes | 143a | 307 | 149 |
| Enriched pathways | Metabolic pathways | Metabolic pathways | Cell cycle |
| Arginine and proline metabolism | Lysosome | Focal adhesion | |
| Sphingolipid metabolism | Peroxisome | ECM-receptor interaction | |
| Protein processing in endoplasmic reticulum | Arginine and proline metabolism | Small cell lung cancer | |
| Protein export | Glycosaminoglycan degradation | Oocyte meiosis | |
| Histidine metabolism | Other glycan degradation | Pathways in cancer | |
| Alanine, aspartate and glutamate metabolism | N-Glycan biosynthesis | Amoebiasis | |
| ABC transporters | Pathways in cancer | Natural killer cell mediated cytotoxicity | |
| Malaria | Valine, leucine, and isoleucine degradation | Bacterial invasion of epithelial cells | |
| Inositol phosphate metabolism | Glycine, serine, and threonine metabolism | p53 signaling pathway | |
| Enriched disease association | Nervous System | Mental retardation | Retinoblastoma |
| Diseases | Metabolism, inborn errors | Osteosarcoma | |
| Mental disorders | Nervous system diseases | Adhesion | |
| Dislocation of hip NOS | Syndrome | Ovarian neoplasms | |
| Respiratory | Metabolic diseases | Neoplasm, residual | |
| Insufficiency | Congenital abnormalities | Drug interaction with drug | |
| Neuromuscular diseases | Protein deficiency | Celiac disease | |
| Xeroderma pigmentosum | Mental retardation, autosomal recessive | Tuberous sclerosis | |
| Muscle rigidity | Neurobehavioral | Cancer or viral infections | |
| Wolfram syndrome | Manifestations | Sarcoma | |
| Cleft palate | Neurologic manifestations | ||
| Central nervous system diseases |
- a Genes with putative pathogenic mutations found in the two probands, including frameshift, stopgain, stoploss, initial codon, splice site, non-synonymous, with pathogenicity score ≥1, and conservation score ≥1, MAF < 1%.
- b Positive control genes: 307 ID genes reported by both Vissers et al. and a website containing known ID gene list (http://gfuncpathdb.ucdenver.edu/iddrc/iddrc/data/iddrcgname.html)
- c Negative control genes: genes randomly extracted from whole human genome by in silico method as described previously (Qiao et al., 2010). Both positive and negative control genes are listed in Supplementary Table S2.
4 DISCUSSION
Our study used exome sequencing to search for genetic causes of phenotypic variability beyond the 1q21.1 CNV. We found no rare deleterious variants in exons on the intact 1q21.1 region, or in the 10 Mb area flanking the CNV locus, among the five CNV carriers from two unrelated families. However, five deleterious varaints were identified in the more severely affected probands, affecting genes with a role in stress-response related processes (Table 1). We previously reported dosage sensitivity of two genes within 1q21.1 CNV region (CHD1L and PRKAB2) with known role in sensing stress in the environment (genomic damage and energy balance, respectively) (Harvard et al., 2011). The exome sequencing based findings therefore increase the number of genes with a role in stress response related processes that are mutated (either due to CNV or sequence changes) in probands of 1q21.1 CNVs.
In the proband of Family A, we found compound heterozygous mutations in SMPD1 which encodes a lysosomal acid sphingomyelinase (ASMase) converting sphingomyelin to ceramide. ASMase was reported to be critical for alcohol-induced hepatic ER stress (Mari, Morales, Colell, Garcia-Ruiz, & Fernandez-Checa, 2014), cellular stress response (Lee et al., 2016), and oxidative stress (Li, Gulbins, & Zhang, 2012). Bi-allelic rare deleterious variants of this gene are frequently found in patients with Niemann-Pick disease (NPD) types A/B (MIMs 2,57,200 and 6,07,616, respectively), lysosomal storage disorders caused by the deficiency of ASMase (Schuchman, 2009) and characterized by ataxia, poor muscle tone, severe liver disease, interstitial lung disease, speech and swallowing defects, progressive decline in intellectual function and seizures. The previously reported mutations do not overlap with our variants, which may explain the difference in phenotypic features. Phenotypic diversity for different mutations in the same gene has been reported previously for other diseases (Cohen, Chinnery, & Copeland, 2010; Sukalo et al., 2014). Biochemical test of acid sphingomyelinase (ASM) activity was not possible because the family was lost to contact. In proband A1, we also found a deleterious hemizygous de novo missense mutation in WNK3. This gene plays multiple physiological roles including sensing osmotic stress, regulation of electrolyte flux, cell growth, differentiation, apoptosis, and cancer (Kahle, Ring, & Lifton, 2008). WNK3 is expressed most highly in the brain and implicated in maintaining cellular volume in response to osmotic stress and regulating neural excitability to GABA (Kahle et al., 2005). Interestingly, the chromosome X carrying the WNK3 mutation was found to be preferentially inactive in the maternal grandmother (A3, who was developmentally normal), but was preferentially active in the mildly affected mother, who transmitted the mutation to her affected son. This suggested that this WNK3 mutation, and in general X-linked mutations in males with 1q21.1 CNV should be considered in conjunction with the chromosome X inactivation patterns, as they may contribute to the variable phenotypes between males and females.
In Family B, we found deleterious mutations in three genes: de novo mutations in EFHC1, paternal heterozygous LOF mutations in NOS1, and ATF6. EFHC1 is involved in oxidative stress by interacting with the redox-sensitive TRPM2 channel which plays a critical role in neuronal cell death (Katano et al., 2012). Heterozygous mutations in EFHC1 have been found in ∼5% of families with Juvenile myoclonic epilepsy (JME) (Suzuki et al., 2004). Brain abnormalities (small hippocampus, enlarged brain ventricles, abnormal brain ependyma motile cilium physiology) and epilepsy were observed in EFHC1 knock-out mice (Suzuki et al., 2009), suggesting the involvement of deleterious variants in this gene in disease pathogenesis. The mutation in proband A was different than the reported variants in patients with Juvenile myoclonic epilepsy. NOS1 synthesizes nitric oxide (NO), which is involved in neurotransmission, and causes neuronal damage elicited by oxidative stress (Scorziello et al., 2004). No human diseases have been linked to variants in this gene, although, association of SNPs in NOS1 with Parkinson disease (Levecque et al., 2003) and schizophrenia (Reif et al., 2006) was reported.
Finally, ATF6 plays a critical role in the unfolded protein response (UPR) to ER stress under different physiological and pathological conditions (Haze et al., 2001), such as ischemia, hypoxia, metabolic factors (glucose starvation, fatty acid levels), and poisons, including neurotoxins (e.g., ethanol) which interfere with normal protein glycosylation and protein folding (Alimov et al., 2013). ATF6 mutations have rarely been reported in humans. A variant in the promoter of ATF6 was associated with psychiatric disorders (Kazeminasab et al., 2012) and homozygous and compound heterozygous variants in ATF6 were reported in individuals with retinal dysfunction (Ansar et al., 2015; Kohl et al., 2015). These reported ATF6 mutations were different than in our patient B. In the mouse knock-out models of Atf6, developmental or brain abnormalities were not reported (Yamamoto et al., 2007), however, neuronal cell death upon insult (ischemia) was increased. This suggested that an ATF6 defect represents a predisposition for an impaired cellular response to stress (Yoshikawa et al., 2015). ATF6α −/− mouse embryonic fibroblasts were reported to have a defect in their ability to induce ER chaperones and Endoplasmatic Reticulum Associated protein Degradation (ERAD) components when challenged by ER stress causing agents, thapsigargin, and tunicamycin (Yamamoto et al., 2007). However, using the same two agents to induce ER stress in our proband's and his father's lymphoblstoid cell lines, we failed to detect any disturbed expression level of several ATF6 target genes. Several factors should be considered in explaining our results. First, EBV transformation may affect the ER stress response in the lymphoblastoid cells as EBV was reported to activate ATF6 (Lee & Sugden, 2008). Second, our experiment was performed at a single time point with fixed concentration of chemical treatment. ATF6 knock-out cells had been demonstrated to respond quickly to short term stress but had a prolonged UPR response to prolonged stress due to the delayed recovery from long term or repeated stress, compared to wild-type cells (Yamamoto et al., 2007). Lastly, the UPR has been reported to be developmentally regulated, that is, the less mature the brain is, the more susceptible it is to ER stress (Wang et al., 2015), and lymphoblastoid cell lines cannot reflect this process. Interestingly, the pool of genes with rare deleterious variants in both probands is enriched in ER stress response and protein export pathways. This pool is also associated with diseases that affect the nervous system, similarly to known ID genes that we studied.
In conclusion, this study represents the first report of exome sequencing in subjects with 1q21.1 CNV and variable phenotypes. Our past and current analysis of genetic abnormalities within and outside of the CNVs point to the involvement of multiple stress response genes and the possible role of the burden of rare deleterious variants that may affect relevant disease pathways. These genetic changes in isolation or combination could make the carriers of the CNVs susceptible to environmental stress which could result in variable neurodevelopmental phenotypes, depending on the severity/timing of the environmental insult. In this model the 1q21.1 CNV alone is not sufficient to cause the abnormal phenotype and the resulting phenotype(s) would be more severe in individuals (e.g., our probands) with 1q21.1 CNVs if they develop in less favorable environments and/or carry additional second hit rare deleterious variants ([Coe, Girirajan, & Eichler, 2012] and Mitchell (2011)], which could also be related to stress response. Sequence analysis of a larger number of families with 1q21.1 CNV would be needed to further analyze the presence of genome wide changes in carriers of 1q21.1 CNV and their impact on gene function and the phenotype.
ACKNOWLEDGMENTS
This work was supported by funding from the Canadian Institutes for Health Research (CIHR) (MOP 74502; PI: ERS). MESL and ERS are Career Scholars supported by the Michael Smith Foundation for Health Research. We also appreciate the support of the family members involved in this study.
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.