Endoplasmic reticulum retention of xylosyltransferase 1 (XYLT1) mutants underlying Desbuquois dysplasia type II
Abstract
Desbuquois syndrome is a heterogeneous rare type of skeletal dysplasia with a prevalence of less than 1 in 1,000,000 individuals. It is characterized by short-limbed dwarfism, dysmorphic facial features, and severe joint laxity. Two types have been recognized depending on the presence of distinctive carpal and phalangeal features. Mutations in the calcium activated nucleotidase 1 (CANT1) have been found to be responsible for type I and lately, for the Kim type of Desbuquois dysplasia. In addition, a number of Desbuquois dysplasia type II patients have been attributed to mutations in xylosyltransferase 1, encoded by the XYLT1 gene, an enzyme that catalyzes the transfer of UDP-xylose (a marker of cartilage destruction) to serine residues of an acceptor protein, essential for the biosynthesis of proteoglycans. We report here a patient with features consistent with Desbuquois dysplasia II including short long bones, flat face, mild monkey wrench appearance of the femoral heads. Whole exome sequencing revealed a novel homozygous duplication of a single nucleotide in XYLT1 gene (c.2169dupA). This variant is predicted to result in a frame-shift and stop codon p.(Val724Serfs*10) within the xylosyltransferase catalytic domain. Immunoflourescence staining of HeLa cells transfected with mutated XYLT1 plasmids constructs of the current as well as the previously reported missense mutations (c.1441C>T, p.(Arg481Trp) and c.1792C>T, p.(Arg598Cys)), revealed aberrant subcellular localization of the enzyme compared to wild-type, suggesting endoplasmic reticulum retention of these mutants as the likely mechanism of disease.
1 INTRODUCTION
Desbuquois dysplasia (DBQD) is an autosomal recessive osteochondrodysplasia characterized by joint laxity, dysmorphic facial features, short extremities, monkey wrench appearance “adjustable wrench with large jaws that has its adjusting screw contained in the handle” of the proximal femur and prominent eyes. DBQD is a heterogeneous syndrome with phenotypes overlapping with Larsen syndrome, spondyloepiphyseal dysplasia, diastrophic dysplasia, and Catel–Manzke syndrome. It has been classified as types I and II based on the presence or absence of characteristic hand anomalies, respectively (Faivre et al., 2004). These anomalies range from accessory ossification center distal to the second metacarpal, delta phalanx, and bifid distal phalanx of the thumb (Bui et al., 2014). Several studies have reported mutations in CANT1 gene to be responsible for DBQD type I (Kim et al., 2010). Although Bui et al. (2014) found that none of their DBQD II patients carried a mutation in CANT1 gene, other studies showed that patients with and without hand anomalies have mutations in this gene. It was therefore concluded that these features are not distinctive criteria to predict the molecular basis of DBQD (Furuichi et al., 2011). In addition, Kim et al. (2010), have described a milder variant of DBQD type I which has also been shown to be caused by mutations in CANT1. This gene encodes a soluble UDP-preferring nucleotidase belonging to the apyrase family involved in cellular signaling.
Recently, patients with DBQD type II have been attributed to mutations in XYLT1 gene encoding xylosyltransferase. XYLT1 (MIM 608124) is located on chromosome 16 (p12.3) and encodes a 959 amino acid protein with a molecular mass of 108 kDa. This enzyme functions as the initiator of the biosynthesis of glycosaminoglycan (GAG) chains, by transferring xylose from UDP-xylose to the core protein to assist in the formation of proteoglycans (PGs) (Bui et al., 2014). The latter is a major constituent of extracellular matrix and is involved in development, scaffolding, and signaling processes (Schreml et al., 2014). In addition, it has been found that loss of GAG chain of PGs can be an early event of osteoarthritis, leading to degradation of cartilage in human, as well as in experimental models (van den Berg, 2011).
Currently, few patients have been identified with homozygous mutations in XYLT1 gene segregating with DBQD type II among different ethnicities, with nonsense, missense, and splice-site mutations (Table 1). However, the underlying cellular mechanisms of disease of most of these mutations have not been fully elucidated.
| DNA change | Protein change | No. of patients | Age (years) | Origin | Consanguinity | Ref. |
|---|---|---|---|---|---|---|
| c.1792C>T | p.Arg598Cys | 2 sibs | 24, 20 | Tunisian | Yes | Bui et al. (2014) |
| c.439C>T | p.Arg147* | 1 | 14 | Mauritian | Yes | Bui et al. (2014) |
| c.276dup | p.Pro93Alafs*69 | 1 | 23 | Belgian | Yes | Bui et al. (2014) |
| c.1588-3C>T | Exon skipping | 1 | 5.5 | Turkish | Yes | Bui et al. (2014) |
| c.1290-2A>C | Exon skipping | 1 | 13 | Turkish | Yes | Bui et al. (2014) |
| c.1290-2A>C | Exon skipping | 1 | 1 | Turkish | Yes | Bui et al. (2014) |
| c.1441C>T | p.Arg481Trp | 2 sibs | 16, 18 | Turkish | Yes | Schreml et al. (2014) |
| c.1651C>T | p.(Arg551Cys) | 1 | 11 | Brazilian | Yes | Silveira, Leal, and Cavalcanti (2016) |
| Compound heterozygous (c.595C>T, c. 1651 C>T) | p.(Gln199*), p.(Arg551Cys) | 1 | 7 | Polish | No | Jamsheer et al. (2016) |
| Compound heterozygous (c.1588-10_1595del, 3.3 Mb del) | 0-alleles | 1 sib, 3 stillbirths | 5 weeks | Dutch | No | van Koningsbruggen et al. (2016) |
We report here the identification of a novel homozygous variant (c.2169dupA) in XYLT1 gene leading to a frame shift p.(Val724Serfs*10) and stop codon in the protein in an Emirati child of consanguineous parents. The affected child was diagnosed with DBQD without hand involvement. In addition, we demonstrate that this mutant as well as two previously reported missense mutants p.(Arg481Trp) and p.(Arg598Cys) are retained in the endoplasmic reticulum (ER) suggesting the ER quality control within this organelle as a major mechanism of disease in DBQD II caused by XYLT1 mutations.
2 MATERIALS AND METHODS
This study was reviewed and approved by Al-Ain Medical Human Research Ethics Committee (AAMD/HREC 10/09). We examined an Emirati child who was referred to Tawam Hospital (Al-Ain) and who fulfilled the criteria for DBQD type II. Informed written consent was obtained from the parents and blood samples were collected from all family members. Genomic DNA was purified from peripheral blood leukocytes following the Flexigene DNA extraction kit protocols (Qiagen. Gmbh, Germany). Mammalian expression vectors were used to express enzymes since fibroblasts from patient could not be obtained.
2.1 Whole exome sequencing (WES) and filtering of the variants
WES was performed for the affected child as a clinical service by the Medical Genetic Laboratories at Baylor College of Medicine (www.bcmgeneticlabs.org). Variants obtained from WES were compared with dbSNP build 147 and the genomic browser databases such as Ensembl (http://ensembl.org), Exome Variant Server (http://gvs.gs.washington.edu/gvs/), the Exome Aggregation Consortium (http://exac.broadinstitute.org), the Arab Genotype Frequency Database (https://galaxc.sengenics.com), and the 1000 genomes project (http://1000genomes.org) to establish the novelty of the identified variant.
2.2 In silico analysis of the identified variant
Bioinformatics analyses for the variant was carried out using Mutation Taster and PolyPhen-2 in order to investigate the possibility of the variant's pathogenicity (Adzhubei et al., 2010; Schwarz, Rodelsperger, Schuelke, & Seelow, 2010). Moreover, the variant was checked for conservation using HomoloGene entry (NCBI).
2.3 Sanger DNA sequencing
The identified putative disease-causing variant was re-sequenced using Sanger sequencing on the affected child and his parents at the Medical Genetic Laboratories of Baylor, College of Medicine (www.bcmgeneticlabs.org) to detect whether the variant segregates with the disease.
2.4 Construction of wild-type and mutant XYLT1 expression vectors
XYLT1 wild-type cDNA (EX-E1329-M10) was purchased from GeneCopoeia (GeneCopoeia, Rockville) (www.genecopoeia.com). The XYLT1 cDNA in this vector is c-terminally tagged with Myc-His. This plasmid was sequenced at our facility prior to any further manipulations to confirm correct sequence using the suggested sequencing primers: Forward: 5′-GCGGTAGGCGTGTACGGT-3′ and Reverse: 5′-GTGGCACCTTCCAGGGTC-3′, using the Big Dye Terminator Kit (Applied Biosystems, California) on 3130xl Genetic Analyzer (Applied Biosystems).
The identified variant (c.2169dupA, p.(Val724Serfs*10)) as well as two previously reported missense mutations (c.1441C>T, p.(Arg481Trp) and c.1792C>T, p.(Arg598Cys)) were generated using XYLT1 (NM_022166.3) cDNA construct as template, by site-directed mutagenesis with Pfu Ultra High-Fidelity DNA polymerase (Stratagene, La Jolla, CA). The following primers were designed using PrimerX (www.bioinformatics.org/primerx/) to generate the required mutations using Stratagene kit (Stratagene):
XYLT1-c.2169dupA-F: 5′ GGGTGATGCCGAAAAAAAGTCTTCAAGATCGCAAG 3′
XYLT-c.2169dupA-R: 5′ CTTGCGATCTTGAAGACTTTTTTTCGGCATCACCC 3′
XYLT1-c.1441C>T-F: 5′ GTGGCG CCTGGGAGATTGGCGGATCCCAGAG −3′
XYLT1-c.1441C>T-R: 5′ CTCTGGGATCCGCCAATCTCCCAGGCGCCAC-3′
XYLT1-c.1792C>T-F: 5′ CCTACCTTCTTTGCCTGCAAGTTTGAAGCC 3′
XYLT1-c.1792C>T-R: 5′ GGCTTCAAACTTGCAGGCAAAGAAGGTAGG 3′
All the generated mutant plasmids were Sanger sequenced to confirm the introduction of the desired mutations.
2.5 Cell lines culturing and transfection
Human embryonic kidney 293 T cells (HEK293T) and HeLa cells were maintained in HyClone™ High Glucose Modified Eagles medium (GE Healthcare Life Sciences, Logan, UT) supplemented with 10% fetal bovine serum, 4 mM L-Glutamine, 4,500 mg/L Glucose, Sodium Pyruvate, and 100 U/ml Penicillin/Streptomycin. HeLa cells used for immunofluorescence assay were seeded on 24-well plate sterilized coverslips, incubated for 24 hr and followed by transfection with the wild and the three generated mutants. On the other hand, HEK293T cells which were used for protein extraction and western blot analysis were seeded with a density of 2.5 × 105cells/well in 6-well plates for 48 hr, transfected with the wild-type and the mutant plasmids p.(Arg481Trp), p.(Arg598Cys), and p.(Val724Serfs*10). Transfection was carried out according to the manufacturer's instructions using the Promega, Madison, WI, FuGENE HD transfection kit.
2.6 Immunofluorescence and confocal microscopy
Transfected HeLa cells were washed three times with PBS, fixed with methanol for 5 min at −20°C, then, they were blocked by 2% BSA for 1 hr. Anti-XYLT1 rabbit polyclonal antibody (dilution: 1:50; Sigma, St. Louis, MO) was added to eight wells as the primary antibody, four of them were incubated with ER marker anti-calnexin goat (C20) polyclonal antibody (dilution: 1:50; Santa Cruz Biotechnology Inc., Dallas, TX) whereas, the rest were incubated with the Golgi marker anti Golgin-97 mouse monoclonal antibody (dilution: 1:100; Cell Signaling Technology, Danvers, MA) and incubated for 1 hr at room temperature in dark. Cells were then washed three times with PBS and incubated with the following respective secondary antibodies for 45 min at room temperature in dark: Anti-rabbit IgG (H + L) F(ab)2 Fragment (Alexa Flour 488 conjugate) (dilution: 1:1000; Cell Signaling Technology, Danvers, MA) to all wells, anti-mouse IgG (H + L) F(ab)2 Fragment (Alexa Flour 555 conjugate) (dilution: 1:1000; Cell Signaling Technology) to four wells, and anti-goat IgG (Alexa Flour 555 conjugate) (dilution:1:1000; Life Technologies, Carlsbad, CA) to the remaining four wells. Subsequently, cells were washed and mounted with immunofluor medium (ICN Biomedicals). Between 100 and 200 transfected cells for each construct were inspected and at least images of 10 cells were acquired using Nikon confocal Eclipse 80I microscope (Japan). The experiment was performed in doublets.
2.7 Protein extraction and western blotting
Forty-eight hours post-transfection, cells were scraped and washed twice with ice-cold PBS. To the cell pellets, 200 μl of ice-cold RIPA lysis and extraction buffer (Thermo Fisher Scientific, Waltham, MA) was added with 10% of protease inhibitor (SIGMAFAST Protease Inhibitor Cocktail Tablets, EDTA-Free; Sigma S8830) then incubated at 4°C on a rotator for 1 hr. Then, the extracts were centrifuged at 4°C on 20,000 RPM for 20 min. Supernatants containing the extracted proteins were then transferred into fresh 0.5 ml tubes. Whole cell lysates were mixed with 4X sample loading buffer (Laemmli buffer) containing β-mercaptoethanol as a reducing agent and SDS, boiled at 98°C for 5 min and separated by 8% SDS–PAGE. Separated proteins were transferred onto nitrocellulose membranes. Later, the membranes were washed with 1X Tris buffered saline and Tween 20 (TBST), then blocked with 5% skimmed milk for 1 hr at room temperature, followed by probing with anti-XYLT1 rabbit polyclonal antibodies (dilution: 1:500; Sigma, St. Louis, MO) and anti-tubulin mouse monoclonal antibodies (dilution: 1:15,000; Santa Cruz Biotechnology Inc., Dallas, Texas) as an internal loading control. The blots were probed with secondary antibodies: goat-anti-rabbit IgG peroxidase (dilution: 1:30,000) and rabbit-anti-mouse IgG peroxidase (dilution: 1:80,000) which were purchased from SIGMA (Sigma, St. Louis, MO,). Finally, the blot was developed by enhanced chemiluminescence (ECL) Pierce substrate reagent (Thermo Fisher Scientific, Waltham, MA) and images were captured by Typhoon FLA 9500 Imager (GE Healthcare Life Sciences, Logan, UT).
3 RESULTS
3.1 Patient report
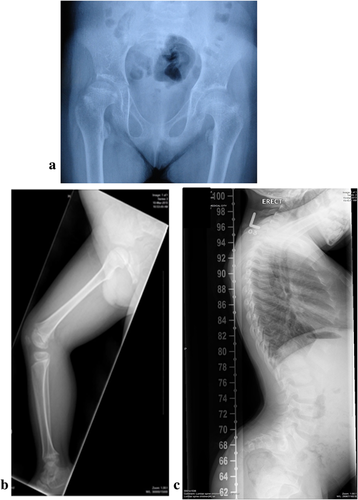
The affected male child was born to first cousin parents of Emirati origin. There was a history of one stillbirth at full term with similar phenotype. The child has one normal sibling and there was no reported history of similar problem in the extended family. The child was conceived through IVF and the pregnancy and delivery at full term were normal. However, prenatal ultrasound at 5 months showed short long bones. His birth weight was 2,440 gm (M = 3.3464, S = 0.14602, L = 0.3487, = > weight z-score: −2.05), length 38 cm (M = 49.8842, SD = 1.8931, (L = 1), = > length z-score: −6.28), and head circumference 33 cm (M = 33.8787, SD = 1.1844, [L = 1) = > HC z-score: −0.74) (http://www.who.int/nutgrowthdb/about/introduction/en/index4.html)(http://www.who.int/growthref/computation.pdf). At birth he was noted to have short limbs, flat face, and small chest. He was evaluated in the Genetic Clinic at the age of 1 month. He had flat face with depressed nasal bridge, long smooth philtrum, small mouth with thin lips, and short neck. Skeletal survey at 1 month showed short femurs with broadening of the proximal and distal metaphyses, bilateral prominence of the minor trochanter “mild monkey wrench appearance,” shortening of the tibia bilaterally with mild bowing and beaks at the metaphyses, shortening and bowing of radius on both sides, the iliac bones were short and broad with shallow acetabulum and flat acetabular roof. There was platyspondyly of the vertebrae with saggital clefts. There was shortening of metacarpal and phalangeal bones with three carpal bones visible and no extra ossification center distal to second metacarpal. Based on these findings, a diagnosis of DBQD without hand involvement was made. Genetic study of CANT1 gene was negative. He was re-evaluated at the age of 8 years and examination showed a weight of 22 kg (M = 25.4163, S = 0.14344, L = −0.5482, = > weight z-score: −1.05), height of 95 cm (M = 127.2651, SD = 5.6480, [L = 1], = > height z-score: −5.71) (http://www.who.int/nutgrowthdb/about/introduction/en/index4.html)(http://www.who.int/growthref/computation.pdf). He had dysmorphic features including flat face with prominent eyes, small mouth, very lax wrist joints and interphallangeal joints, and hyperlordosis. He had normal developmental milestones and was attending normal school. Skeletal survey at 8 years of age showed similar changes as previously observed (Figure 1a–c).
3.2 WES and sanger sequencing revealed a homozygous duplication mutation in (XYLT1) gene
To exploit next generation sequencing, WES analysis was carried out which resulted in the identification of a novel homozygous variant, c.2169dupA which resides in exon 10 in XYLT1 gene in the affected child. This variant was re-sequenced using Sanger sequencing in the affected child and his parents and was found to be homozygous in the affected child and heterozygous in both parents (Supplementary Figure S1a). This variant is not present in the Exome Variant Server database (http://gvs.gs.washington.edu/gvs/). In addition, two more constitutional variants exist (c.2169A>T and c.2169A>G) (rs753887357), with protein change p.(Lys723Asn) and p.(Lys723 =), respectively. However, the minor allelic frequency of them is 0.0008% and the missense variant c.2169A>T, p.(Lys723Asn) was predicted to have a tolerated consequence on the protein.
In our patient, the inserted nucleotide is predicted to disrupt the translational reading frame of the protein leading to the generation of a frame-shift and a termination stop codon at position 732, thus deleting approximately a quarter of the XYLT1 protein (http://expasy.org).
In addition, multiple alignments using Clustal Omega showed that the valine residue is conserved at position 724 among multiple species (Supplementary Figure S1b). In silico analyses using Mutation Taster showed that the variant is disease causing. Furthermore, PolyPhen software predicted the variant to be possibly damaging with a score of 0.732. The expected effects of this variant on the size of the expressed protein was established by introducing the change into the cDNA in mammalian expression vector then expressing the protein in HEK293T cells. As predicted the size of the expressed protein was smaller (∼75 kDa) than the wild-type (∼108 kDa) as shown in the supplementary Figure S1c, confirming truncation of the mutant protein.
3.3 Confocal microscopy suggests ER retention as a cellular mechanism underlying several XYLT1 mutants causing DBQD type II
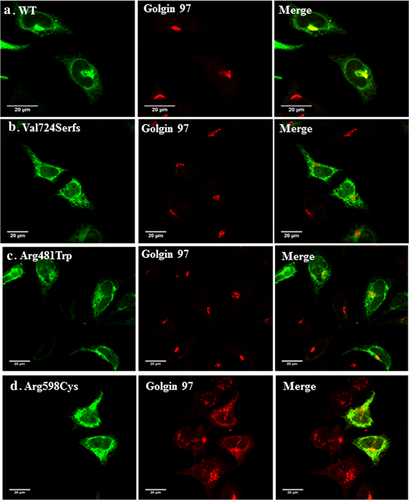
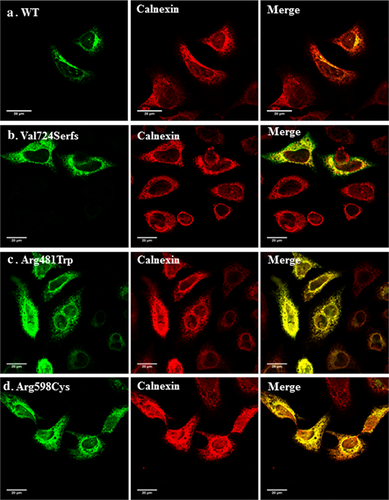
To test the cellular trafficking properties of some DBQD type II-causing mutants, we generated three pathogenic mutations in XYLT1 cDNA cloned into a mammalian expression plasmid as detailed in the Methods section. This included the duplication leading to a frame-shift and stop codon found in our patient and two previously reported missense mutations p.(Arg481Trp) and p.(Arg598Cys). The subcellular localization of the generated XYLT1 mutants was detected by confocal immunofluorescence staining of the transfected HeLa cells, using polyclonal antibodies directed toward the XYLT1 protein. As expected, the wild-type XYLT1 protein localized predominantly to the Golgi apparatus as evidenced by its extensive co-localization with the Golgi marker Golgin-97. In contrast, the three tested mutants did not co-localize with this Golgi marker but showed a more extensive reticular pattern suggesting ER retention (Figure 2). We therefore, co-stained the expressing cells with the well-established ER marker (Calnexin) which showed that the three mutants are largely co-localizing with it in contrast to wild-type (Figure 3) suggesting trafficking defects of the mutant proteins and their retention by ER protein quality control mechanisms.
4 DISCUSSION
DBQD is a rare disease which is part of a group of disorders which are characterized by multiple joint dislocations and therefore given the term “The multiple dislocation group of disorders.” The main features of these bone dysplasias include flat face with prominent eyes, joint laxity with dislocations of large joints, and growth retardation (Alanay & Lachman, 2011). Radiologically, it is characterized by short long bones with a monkey wrench appearance of the proximal femora and advanced carpal and tarsal ossification. The presence of additional hand anomalies (extra ossification center distal to the second metacarpal, delta phalanx, and bifid distal phalanx of the thumb) in some patients has led to its classification into DBQD type I (with additional hand anomalies) and DBQD type II (without such anomalies) (Faivre et al., 2004; Kaissi, Radler, Klaushofer, & Grill, 2009). The patient in this report was classified as DBQD type II, as he had most of the clinical and radiological features of DBQD including the mild monkey wrench abnormality of the femora, but the typical hand anomalies seen in type I were absent. WES analysis on this child revealed a novel variant in XYLT1 gene (c.2169dupA, p.(Val724Serfs*10)) resulting in truncation of the xylosyltransferase enzyme. This variant, segregated with the phenotype among the family members and is expected to result in a truncation and immunofluorescent experiments demonstrated mis-localization resulting in a loss of XYLT1 function which is the first limiting step among a series of enzymes (β1,4 galactosyltransferase1, β1,3 galactosyltransferase2, and β1,3 glucosyltransferase 1) that are spatially related in glycosylation at the Golgi apparatus (Schreml et al., 2014).
During bone development, chondrocytes are surrounded by PG rich matrix that assists in their maturation, in addition to initiating endochondral bone formation. Defects in chondrocytes formation can lead to skeletal phenotype (Eames et al., 2011). PGs synthesis is initiated by XYLT1 enzymes through adding GAG chains covalently, giving PGs the physiochemical features to withstand compression forces (Schreml et al., 2014). Polymorphisms in XYLT1 gene correlated with decreased GAG levels (Ambrosius, Kleesiek, & Götting, 2009). Hence, XYLT1 enzymes are expressed in chondrocytes during fetal development, and in children during mineralization of osteoblasts (Prante, Kuhn, Kleesiek, & Götting, 2009), aside from that, they localize at cis-Golgi where they exert their catalytic function (Mis et al., 2014). Furthermore, reduction in the activity of XYLT1 was associated with a decremental growth of vertebral bones in animal models (Morriss, Fitzgerald, & Riddle, 1985).
Functional study confirmed the mis-localization of the mutant enzyme and its retention into the ER (Figures 2 and 3). This result was also noticed for a missense previously reported mutation p.(Arg481Trp) introduced in this study and which coincides with the finding of Schreml et al. (2014) done on patients’ fibroblasts showing loss of predominance of mutated XYLT1 in Golgi. Likewise, the second generated missense mutation p.(Arg598Cys) which is located in the xylosyltransferase domain and which was expected to be damaging being replaced by a neutral amino acid (Bui et al., 2014), revealed the apparent retention of the enzyme in the ER. We have recently shown that retention in the ER is involved in the cellular mechanism of several musculoskeletal abnormalities including Robinow syndrome (Ali et al., 2007), acromesomelic dysplasia-type Maroteaux (Hume et al., 2009). Bioinformatic analysis performed on our proband predicted the termination to occur at position 732 deleting around 227 amino acids at the c-terminal end of the protein including an important motif (745DWD747) which was shown to be essential for the activity of XYLT1 (Götting, Kuhn, Zahn, Brinkmann, & Kleesiek, 2000), unlike the N-terminus of XYLT1 which was proved not to be involved in the function of UDP or heparin sulfate binding (Müller et al., 2006). Moreover, XYLT1 has three N-glycosylation sites; N-226, N-421, N-777. Loss of the last glycosylation site due to truncation might affect the protein's stability and folding. XYLT1 enzyme is composed of two domains, the glycosyltransferase domain (amino acids: 328–581) and the xylosyltransferase domain (amino acids: 613–794). Thus, the truncation here will clearly omit most of the xylosyltrasnferase activity of the enzyme (Bui et al., 2014). Figure 4 represents the position of our proband variant (c.2169dupA, p.(Val724Serfs*10) as well as all the previously reported mutations relative to both domains.
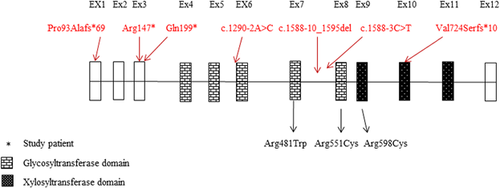
Schreml et al. (2014) studied XYLT1 expression in patient's fibroblasts of the variant (c.1441C>T, p.(Arg481Trp)) and found that there was no apparent reduction in the enzyme expression. However, it led to defects in PG synthesis. On the other hand, the variant (c.1290-2A>C) which affected a donor splice-site in two unrelated Turkish families exhibited reduction in XYLT1 expression as well as, a decrease in chondroitin-sulfate PG (CS-PG). Similarly, the Mauritian family with one affected individual with the variant (c.439C>T, p.(Arg147*), revealed a reduction in CS-PG as well as XYLT1 and two expression, the latter being a paralog to XYLT1 with high degree of homology (Bui et al., 2014).
In summary, we have identified a novel disease-causing XYLT1 variant (c.2169dupA, p.(Val724Serfs*10)) and presented data to suggest that retention of variant XYLT1 protein is the mechanism for decreased XYLT1 activity of several mutations in this gene. Hence, decrease in mature GAGs is the underlying disease mechanism for the disease, and this finding might be considered a functional test in DBQD II patients associated with missense mutations of unknown significance, to confirm pathogenicity.
ACKNOWLEDGMENTS
We thank the family for providing samples in this study. We are also thankful to the United Arab Emirates University for funding this project and supporting NKA PhD studies (Grant 31M144 and 31M241).
CONFLICT OF INTEREST
The authors declare no conflict of interest.