Functional monosomy of 6q27-qter and functional disomy of Xpter-p22.11 due to X;6 translocation with an atypical X-inactivation pattern
Abstract
Pattern of X chromosome inactivation (XCI) is typically random in females. However, chromosomal rearrangements affecting the X chromosome can result in XCI skewing due to cell growth disadvantage. In case of an X;autosome translocation, this usually leads to an XCI pattern of 100:0 with the derivative X being the active one in the majority of females. A de novo balanced X;6 translocation [46,X,t(X;6)(p22.1;q27)] and a completely skewed XCI pattern (100:0) were detected in a female patient with microcephaly, cerebellar vermis hypoplasia, heart defect, and severe developmental delay. We mapped the breakpoint regions using fluorescence in situ hybridization and found the X-linked gene POLA1 to be disrupted. POLA1 codes for the catalytic subunit of the polymerase α-primase complex which is responsible for initiation of the DNA replication process; absence of POLA1 is probably incompatible with life. Consequently, by RBA banding we determined which of the X chromosomes was the active one in the patient. In all examined lymphocytes the wild-type X chromosome was active. We propose that completely skewed XCI favoring the normal X chromosome resulted from death of cells with an active derivative X that was caused by a non-functional POLA1 gene. In summary, we conclude that functional monosomy of 6q27-qter and functional disomy of Xpter-p22.11 are responsible for the clinical phenotype of the patient. This case demonstrates the importance of determining which one of the X chromosomes underwent inactivation to correlate clinical features of a female with an X;autosome translocation with the nature of the genetic alteration.
1 INTRODUCTION
The clinical consequences of chromosomal translocations depend on whether any genetic information is affected by them. It can be distinguished between balanced and unbalanced translocations. Balanced translocations lead to no gain or loss of genetic material and therefore are often silent and do not cause any clinical phenotype (Kloosterman & Hochstenbach, 2014). However, a balanced chromosomal translocation can be pathogenic if a break disrupts a gene or a regulatory element or separates them from each other. Also, the transfer of a gene into a heterochromatin area means its inactivation and can consequently lead to a clinical picture without changing the gene dosage (Kleinjan & Lettice, 2008; Kleinjan & van Heyningen, 2005). Apparently, balanced translocations can be associated with genomic imbalances such as cryptic deletions and/or duplications that are detectable by array CGH (Schluth-Bolard et al., 2009). Indeed, submicroscopic deletions have been identified in 30–50% of apparently balanced translocations associated with a disease phenotype (Baptista et al., 2008; De Gregori et al., 2007; Gribble et al., 2005). In X;autosome translocations, incorrect dosage of both autosomal and X-linked genes can occur due to preferential X chromosome inactivation (XCI) of the derivative X [der(X)] chromosome. Inactivation of the der(X) that contains the autosomal fragment leads to functional monosomy of the translocated autosomal genes and to functional disomy for the portion of the X chromosome onto the active derivative autosome. However, there is strong selection against such cells leading to completely skewed XCI in the majority of cases with the der(X) being active and the normal X chromosome being inactive (Kalz-Fuller, Sleegers, Schwanitz, & Schubert, 1999; Schluth et al., 2007; Schmidt & Du Sart, 1992). In the vast majority of females, a skewed XCI pattern does not lead to any clinical anomalies. However, if the translocation causes a gene disruption on the der(X), the lack of that gene product can induce a clinical phenotype (Tommerup, 1993). Importantly, interruption of an X-linked gene required for cell growth or survival can lead to post-inactivation cell selection mechanism and an XCI pattern with an active normal X and an inactive derivative X chromosome. For example, disruption of the X-linked DKC1 gene in a girl with X;1 translocation caused an atypical XCI with functional disomy of Xq28 that likely was associated with her clinical abnormalities (Cottrell et al., 2009). Here we report on a girl with an X;6 translocation and an unusual XCI that resulted in functional disomy of Xpter-p22.11 and functional monosomy of 6q27-qter.
2 CLINICAL REPORT
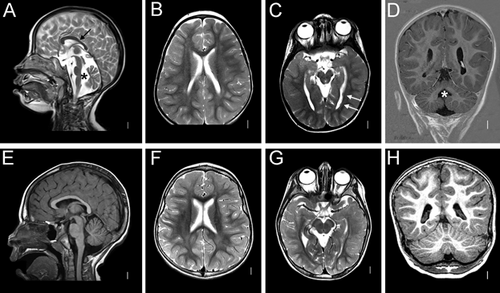
The patient was admitted for genetic counseling due to severe developmental delay and microcephaly. Family history was unremarkable. At the time of conception, her mother was 24 and her father 23 years old. She was born after an uneventful pregnancy at 37th week of gestation with nearly normal birth measurements (weight 3600 g [−1.43 SD], length 48 cm [−0.63 SD], OFC 31 cm [−2 SD]). Ventricle septal defect was diagnosed shortly after birth and surgically corrected during the second year. At the first visit at 2 years and 6 months, she was not able to sit and walk and had no speech development. Additionally, she showed bilateral hip dislocations, which were operated at the age of 5 years. Facial dysmorphism included bilateral epicanthic folds, wide nasal bridge, broad nasal tip, broad philtrum, thick and everted lower lip, and hypotonic face with mouth held open and reduced facial expression (Figure 1). Cranial magnetic resonance imaging at 4 years revealed cerebellar vermis hypoplasia and two unilateral periventricular nodular heterotopias (Figure 2). During the last follow-up at 7 years and 10 months, her height was 118 cm (–1.6 SD), weight 18 kg (–2.3 SD), and OFC 46 cm (–4 SD). She could independently sit, crawl, and get on her knees. She did not develop any active speech. The girl had severe feeding difficulties and could eat only pureed food.

3 METHODS
Chromosomal analysis of peripheral blood lymphocytes was performed according to routine procedures using GTG-banding at approximately 550 band resolution per haploid set. Oligonucleotide array CGH using the Human Genome CGH Microarray Kit 244A was performed on leukocyte-derived genomic DNA extracted with the QIAamp DNA Blood Maxi Kit (Qiagen, Hilden, Germany) following the manufacturer's recommendations (Agilent, Santa Clara, CA), with 1.5 μg genomic DNA used for labeling. Commercially available female DNA pool was used as a control (G1521 Promega, Mannheim, Germany). An Agilent DNA microarray scanner (G2505C) was used and normalization was carried out with standard settings of the Feature Extraction software version 9.5. Data analysis was performed with Agilent's Genomic Workbench 5.0.14 with the NCBI36/hg18 assembly as reference genome. Genomic coordinated were later converted to GRCh37/hg19. The ADM-2 algorithm was applied to calculate genomic imbalances. A minimum of three consecutive probes had to be affected for a call. The threshold was set to 5.9.
Metaphase spreads from peripheral blood lymphocytes were prepared by standard procedure and counterstained using 4′,6-diamidino-2-phenylindole (DAPI). BAC (RP11 human BAC library) clones were received from the BACPAC Resource Center, Children's Hospital Oakland, California. BAC DNA preparation and labeling as well as fluorescence microscopy and analysis of images was performed as previously described (Chilian et al., 2013). Fluorescence in situ hybridization (FISH) using BACs RP11-730G11 (chr6:107,450,040–107,612,875) and RP11-794N2 (chr6:107,621,685–107,821,156) (NCBI37/hg19) located in 6q21 was used for confirmation of the array result and for analysis of the healthy parents. FISH was also applied for breakpoint mapping of the X;6 translocation. We used 10 BACs located in and around the breakpoint at Xp22.11 and 8 BACs located at 6q27 (Supplementary Table 1).
The SOBP and PDSS2 genes were analyzed by Sanger-sequencing of the coding region and flanking intronic sequences. The analysis was done by an external certified lab provider.
Late replication study to distinguish between the active (Xa) and inactive (Xi) X chromosome was performed on lymphocytes by R-banding BrdU acridine orange (RBA) technique as described previously (Dutrillaux, 1976; Verma & Lubs, 1975). After cell synchronization with methotrexate (MTX) and 5 hr before harvest we added BrdU to a final concentration of 100 μM. Air-dried slides were stained with 10% acridine orange for 50 sec, rinsed in phosphate buffer (Sorensen's buffer, pH 6.8), mounted in buffer, and analyzed under a fluorescence microscope (DMR, Leica Microsystems, Wetzlar, Germany). This method resulted in R-banding with a readily distinguishable late replicating Xi from an early replicating Xa. In total, 20 metaphases at the 500–550 band level were analyzed.
4 RESULTS
Chromosomal analysis in the patient revealed an apparently balanced reciprocal translocation with the karyotype 46,X,t(X;6)(p22.1;q27) (Figure 3A). Both parental karyotypes were normal, suggesting that the translocation occurred de novo in the patient (data not shown). Chromosomal microarray revealed no copy number variations (CNVs) at Xp22.1 and 6q27; however, a microdeletion at 6q21 of about 352 kb (arr[hg19] 6q21(107,568,491–107,920,960)x1) encompassing the genes PDSS2 and SOBP was detected (data not shown). FISH analysis confirmed the deletion in the patient and showed absence of the CNV in the parents (data not shown). This microdeletion is absent from the Database of Genomic Variants and from any other database. Biallelic mutations in PDSS2 cause one of the autosomal recessive forms of the Leigh syndrome (Lopez et al., 2006), and mutations in the SOBP gene are associated with the MRAMS (mental retardation, anterior maxillary protrusion, strabismus) syndrome (Basel-Vanagaite et al., 2007; Birk et al., 2010). Sanger sequencing of SOBP and PDSS2 did not disclose any mutation on the second allele of the patient (data not shown). We concluded that the 6q21 microdeletion is unlikely to be causative for the patient's phenotype and proceeded with breakpoint mapping of the X;6 translocation.
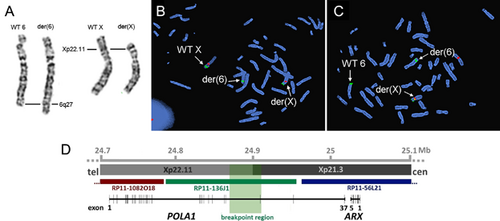
The first FISH cycle showed that the breakpoint on the X chromosome and chromosome 6 is situated between RP11-65K7 and RP11-645G16 and RP11-235F13 and RP11-673P11, respectively (Supplementary Figure 1). The second cycle with the BACs located between these boundaries revealed two probes giving a split signal: RP11-136J1 in Xp22.11 and RP11-757H20 in 6q27 (Figure 3B and C).
RP11-136J1 spans a large region of the gene POLA1, which encodes the catalytic subunit p180 of the DNA polymerase α (Polα) (Figure 3D). Polα is required for DNA replication, in particular for priming of Okazaki fragments (Forterre, 2013). RP11-757H20 covers the PRR18 gene and partly the SFT2D1 gene (Supplementary Figure 1).
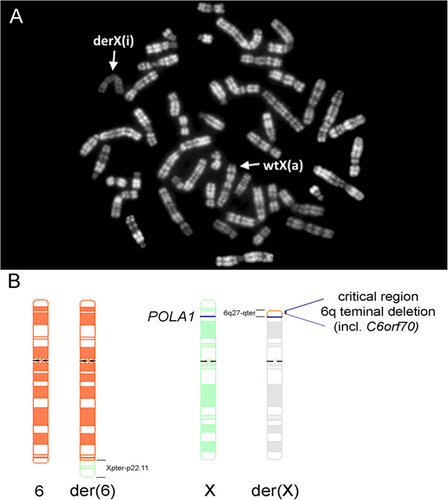
To our surprise and contrary to the expectation, RBA staining demonstrated that not the normal X chromosome but the der(X) was inactivated in 100% of the studied cells (Figure 4A).
5 DISCUSSION
We report on a female patient with multiple congenital anomalies and severe intellectual disability (ID) carrying a de novo reciprocal translocation: 46,X,t(X;6)(p22.1;q27). Array CGH did not reveal any genomic imbalance associated with her phenotype. Breakpoints at Xp22.1 and 6q27 were delineated to potentially uncover disease-associated gene disruption and a novel gene associated with a syndromic ID form. We identified disruption of the POLA1 gene on the X chromosome and possible disruption of PRR18 and/or SFT2D1 within the breakpoint at 6q27. None of the three genes has been linked to any human disorder so far.
5.1 The POLA1 gene
POLA1 encodes the catalytic subunit p180 of the Polα which is part of the replication machinery in eukaryotes and the only enzyme able to start DNA synthesis de novo (Muzi-Falconi, Giannattasio, Foiani, & Plevani, 2003; Pellegrini, 2012). Thus, it plays a central role in the process of genomic duplication and its function cannot be replaced by other polymerase isoforms (Zhang, Baranovskiy, Tahirov, & Pavlov, 2014). However, Polα is also involved in other cellular processes, such as response to DNA damage, telomere maintenance, and epigenetic control of chromatin structure (Muzi-Falconi et al., 2003). These data suggest that the POLA1 gene is essential for cell survival, and absence of POLA1 protein might be incompatible with life. Consequently, disruption of POLA1 by the Xp22.11 breakpoint most likely resulted in a cell selection mechanism during early embryogenesis causing survival of cells with the normal X chromosome active and demise of cells with the derivative X chromosome being the active one. Thus, skewing of XCI in our patient preserved POLA1 expression from the wild-type X, but most likely caused silencing of genes in the ∼4.5-Mb interval of 6q27 due to spreading of inactivation from the adjoining X chromosome. This scenario is in line with the hypothesis put forward by Wang et al. (1985) that deletion of POLA1 would be lethal for the cell and lead to a completely skewed XCI in females (Wang et al., 1985). Several papers reported deletions at Xp22.11 ranging from ∼50 to ∼400 kb in males (Filges et al., 2011; Noor et al., 2010; van Kogelenberg et al., 2011). However, none of the reported Xp22.11 deletions encompasses POLA1 (Supplementary Figure 2). Given the functional importance of the POLA1 protein, these findings might indicate that human male embryos who harbor a defective POLA1 gene or even lack it are not viable and die early in utero.
5.2 Xpter-p22.11 duplication and 6q terminal deletion syndrome
The skewed XCI pattern in our patient probably resulted in functional disomy of Xpter-p22.11 and functional monosomy of 6q27-qter (Figure 4B) that likely are associated with the clinical features of the girl.
Partial duplications in either arm of the X chromosome are rare and difficult to interpret. However, previous work has provided evidence that increased copy number of genes on the X chromosome can contribute to deregulation of normal cognitive development (Froyen et al., 2007). Several males harboring Xpter-p22.11 (micro)duplications ranging from 41 kb to ∼12.5 Mb (Gijsbers et al., 2011; Honda et al., 2010; Wagenstaller et al., 2007; Whibley et al., 2010) as well as patients with a cytogenetically visible duplication >15 Mb have been reported (Telvi et al., 1996). Phenotypes were rather unspecific encompassing moderate-to-severe ID, speech delay, behavior anomalies with jerks and stereotypic movements, autistic features, West syndrome, and occasional but not overlapping congenital anomalies (microcephaly, cleft palate, persistent ductus arteriosus). The majority of the duplications was inherited from the unaffected mother. More than 50 male and female patients with an Xp22.31 microduplication involving the STS gene have been reported to date (Esplin et al., 2014). This locus is discussed as a risk factor for an abnormal phenotype (Faletra et al., 2012; Li et al., 2010; Liu et al., 2011). The patients had non-syndromic ID, feeding difficulties, autism spectrum disorders, and hypotonia as common features. In symptomatic female carriers functional disomy of genes within the duplication as a result of an unfavorable XCI has been hypothesized to be causative for their neurodevelopmental phenotype. However, the phenotypic spectrum associated with Xp22 duplications in females and the precise role of XCI could not be defined so far (Esplin et al., 2014).
6q terminal deletion syndrome is caused by subtelomeric deletion of 6q26-qter and associated with a range of developmental brain abnormalities including hydrocephalus, corpus callosum and cerebellar vermis dysgenesis, colpocephaly, polymicrogyria (Eash, Waggoner, Chung, Stevenson, & Martin, 2005; Elia et al., 2006; Stevenson et al., 2004; Striano et al., 2006), and periventricular nodular heterotopia (PNH) (Bertini, De Vito, Costa, Simi, & Valetto, 2006; Dobyns et al., 2008). Conti et al. (2013) identified the minimal critical region of 1.2 Mb associated with the complex brain phenotype and demonstrated that haploinsufficiency of C6orf70 is responsible for PNH. The majority of patients presented with developmental delay or ID (Backx et al., 2010; Gerber, Neuhann, Tyshchenko, Smitka, & Hackmann, 2011); however, most of them were mildly to moderately affected (Conti et al., 2013). Brain imaging data of our patient are fully compatible with the described MRI findings of patients with the 6q terminal deletion syndrome that are cerebellar vermis dysgenesis and PNH (Conti et al., 2013). In addition, facial dysmorphism in our patient such as bilateral epicanthic folds, broad philtrum, and thick lower lip have repeatedly been described in patients with 6q terminal deletions (Conti et al., 2013). Although the majority of clinical features in the reported girl are similar to those of patients with 6q deletion syndrome and can thus be attributed to functional 6q27-qter monosomy, we cannot exclude a combined impact of both functional chromosomal anomalies on the patient's phenotype.
5.3 XCI in X;autosome translocation
To our knowledge, an X;autosome translocation that goes along with X-chromosomal gene disruption and a completely skewed XCI pattern comprising 100% of cells with an active normal X has been described only twice (Cottrell et al., 2009; Gläser et al., 2004). Gläser et al. (2004) reported a girl with t(X;15)(p11.3;q26) and multiple congenital malformations. The authors suggested that disruption or disturbance of the X-chromosomal KIAA0215/JADE3 gene which encodes a plant homeo-domain bearing protein functioning in histone H4 acetylation (Panchenko, 2016) caused death of cells with an active derivative X chromosome (Gläser et al., 2004). In the case reported by Cottrell et al. (2009), the X-chromosomal breakpoint disrupted the DKC1 gene which encodes the dyskerin protein that participates in multiple nuclear protein complexes (Angrisani, Vicidomini, Turano, & Furia, 2014). The atypical X inactivation pattern in this patient resulted in functional Xq28 disomy. An important difference to our case is, however, that the X;1 translocation concerned a very large autosomal segment (∼93.9 Mb) comprising almost the whole q arm whose inactivation would have been lethal. Cottrell et al. (2009) showed that the translocated autosomal part was not inactivated by the joined X chromosome and the clinical phenotype of the reported patient was likely due to functional disomy of the X-chromosomal segment (Cottrell et al., 2009). Similarly, functional Xp disomy has also been described in a girl with developmental delay and the karyotype 46,X,t(X;13)(p11.2;p13). She also had skewed XCI with a preferentially inactivated derivative X chromosome, however, the breakpoint at Xp11.2 has not been fine-mapped in this case (Myszka et al., 2010). In our case, RBA banding did not show any observable bright region at the tip of the p arm of the rearranged X suggesting that the translocated terminal part of 6q was inactivated (Figure 4A).
Taken together, we demonstrate that X;autosome translocations can result in completely skewed X-inactivation patterns in favor of an active wild-type X chromosome. This possibility must be considered when investigating X;autosome translocations, especially when the translocated autosomal region on the der(X) is rather small (comprising a few Mb). Clinical phenotypes in such translocation carriers can therefore be a consequence of functional disomy of X-chromosomal DNA, functional monosomy of silenced autosomal genes on the inactive der(X) or of both.
ACKNOWLEDGMENTS
We are grateful to the patient and her family for participating in this study. We thank Jennifer Kaiser for skillful technical assistance. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (KU 1240/5-1 to K.K.).
CONFLICT OF INTEREST
None.