TSC2 c.1864C>T variant associated with mild cases of tuberous sclerosis complex
Abstract
Tuberous sclerosis complex (TSC) is an autosomal dominantly inherited disorder with variable expressivity associated with hamartomatous tumors, abnormalities of the skin, and neurologic problems including seizures, intellectual disability, and autism. TSC is caused by pathogenic variants in either TSC1 or TSC2. In general, TSC2 pathogenic variants are associated with a more severe phenotype than TSC1 pathogenic variants. Here, we report a pathogenic TSC2 variant, c.1864C>T, p.(Arg622Trp), associated with a mild phenotype, with most carriers meeting fewer than two major clinical diagnostic criteria for TSC. This finding has significant implications for counseling patients regarding prognosis. More patient data are required before changing the surveillance recommendations for patients with the reported variant. However, consideration should be given to tailoring surveillance recommendations for all pathogenic TSC1 and TSC2 variants with documented milder clinical sequelae. © 2017 Wiley Periodicals, Inc.
INTRODUCTION
Tuberous sclerosis complex (TSC) is an autosomal dominantly inherited disorder with variable expressivity associated with hamartomatous tumors, abnormalities of the skin, and neurologic problems including seizures, intellectual disability (ID), and autism [Northrup et al., 2015]. TSC is caused by inactivating variants in the TSC1 and TSC2 tumor suppressor genes, encoding essential components of the TSC complex, an important negative regulator of the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1). A definite clinical diagnosis of TSC can be made if a person has at least two major features, or one major feature and two minor features of the disease. At the 2012 International TSC Clinical Consensus Conference, the diagnostic criteria were revised to account for molecular genetic testing, specifying that identification of a pathogenic TSC1 or TSC2 variant is sufficient for diagnosis [Northrup and Krueger, 2013]. Genetic testing is typically utilized for family planning and to aid in the diagnosis of patients suspected of having TSC, but who do not meet the criteria for a definite clinical diagnosis. Currently, because of the widely variable phenotypic expression and lack of genotype–phenotype correlations in TSC, the results of genetic testing do not typically change disease management and offer minimal prognostic information.
Here, we report a series of patients with a pathogenic TSC2 c.1864C>T p.(Arg622Trp) variant and a mild TSC phenotype, with most patients not meeting the clinical diagnostic criteria for definite TSC. Our study has significant implications for prognosis, and may lead to changes in management and/or disease classification for these individuals.
CLINICAL REPORT
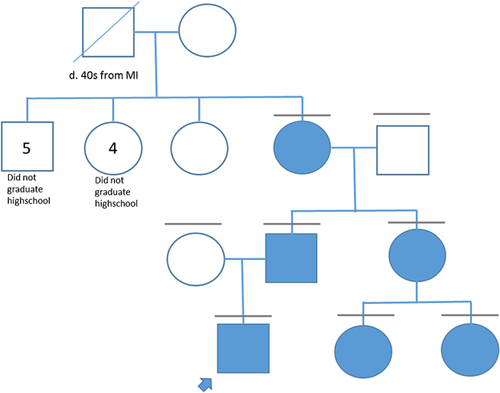
Patient 1 (IV-1, Fig. 1) was followed since birth for prenatally detected and postnatally verified cardiac rhabdomyomas. Genetic testing for TSC was performed due to the presence of rhabdomyomas. TSC1 sequence analysis, and TSC1 and TSC2 deletion/duplication testing were normal. Sequence analysis of TSC2 identified a c.1864C>T, p.(Arg622Trp) variant. The variant was also found in the proband's father (III-2), paternal grandmother (II-4), paternal aunt (III-3), and paternal 2 years old (IV-2) and newborn cousins (IV-3) (Fig. 1).
At age 3 years, the proband was developmentally normal with no stigmata of TSC aside from a regressing rhabdomyoma. Physical examination, including Wood's lamp exam, did not find evidence for hypopigmented macules, dental pits, oral or ungual fibromas, shagreen patches, or angiofibromas. Neurologic and ophthalmologic exams were normal. Developmental evaluations performed as part of a research study were normal. Specifically, composite scores of overall development on the Mullen Scales of Early Learning were 104, 109, and 111 at 12, 24, and 36 months, respectively (average range 85–115). Composite scores of receptive and expressive language skills on the Preschool Language Scale-5 were 93, 120, and 110 at ages 12, 24, and 36 months, respectively (average range 85–115). Annual abdominal imaging, brain MRIs, and EEGs were normal.
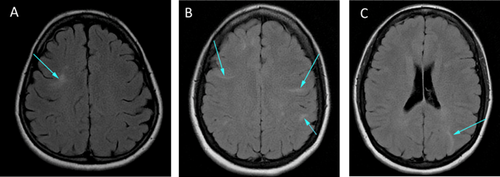
Other family members who carry the variant were evaluated for TSC-associated findings. There was no history of seizures or ID in any family member. The father reported academic difficulty and the paternal grandmother did not complete high school, but both were of normal intelligence. The paternal aunt had no learning difficulties. Physical examinations for cutaneous findings of TSC were negative (WG and HN). The father did not undergo imaging. The paternal grandmother had scattered cortical tubers and findings consistent with mild microangiopathic changes (Fig. 2). Abdominal MRI demonstrated two small renal cortical cysts at age 59. The 37-year old paternal aunt had multiple radial migration lines and small cortical tubers (Fig. 2), no CT findings of lymphangioleimyomatosis, a simple hepatic cyst (2.9 × 2 cm), and a single renal cyst (3 mm). Her two daughters (IV-2 and IV-3; Fig. 1) also have the variant. The older child (IV-2; Fig. 1) had a hyperdense area adjacent to the left atrioventricular groove on echocardiogram, but no external findings of TSC (WG) and no other imaging. The younger child (IV-3; Fig. 1), a newborn, had four cardiac rhabdomyomas, normal head ultrasound, normal abdominal ultrasound, and no cutaneous findings of TSC (WG). At age 12 months, all but one rhabdomyoma had regressed (Suppl. Table SI).
Patient 2 was diagnosed with probable TSC based on the presence of cortical tubers and renal cysts. She presented at age 7 when she was hospitalized for an infection of the urinary tract and was found to have polycystic kidneys and hypertension with normal renal function. She had a history of migraines, attention deficit hyperactivity disorder, and required extra help in school. Brain imaging identified a single cortical tuber. TSC2 testing identified the c.1864C>T variant. Given the significant cystic kidney disease, PKD1 and PKD2 testing were also performed. No pathogenic variants were detected, but three variants of unknown significance (VUS) in PKD1 were identified. At age 15, she had no cutaneous manifestations, renal angiomyolipomas, subependymal nodules (SENs), subependymal giant cell astrocytomas (SEGAs), rhabdomyomas, or seizures (Suppl. Table SI).
Patient 3 was identified with rhabdomyoma at age 3 months and required surgical intervention at age 5 months. An asystolic event at 1 year resulted in hypoxic brain injury, leading to severe ID. Prior to this episode, there was no developmental delay. At age 1, there were no other stigmata of TSC. Brain imaging demonstrated ischemia of the gray matter and widening of the ventricles, consistent with hypoxic brain injury, but no stigmata of TSC. Genetic testing identified the TSC2 c.1864C>T variant. The parents were not evaluated for the variant. They had normal eye and Wood's lamp examinations, and normal brain and renal imaging. The patient had a healthy older sister and younger brother. Patient 3 was institutionalized and expired at 8 years from gastrostomy tube complications (Suppl. Table SI).
Patients 4 and 5 are siblings with rhabdomyoma. They inherited the TSC2 c.1864C>T variant from their mother. At age 8, patient 4 had normal development, cortical dysplasias, and one <0.5 cm hypomelanotic macule. Patient 5, a newborn, had rhabdomyoma as her only feature. The mother had no cutaneous findings and normal renal/lung imaging (Suppl. Table SI).
Patient 6 is a 2-year-old female with rhabdomyoma and no other findings of TSC. She inherited the TSC2 c.1864C>T variant from her mother who has hyperintensities on brain MRI but no other findings of TSC (Suppl. Table SI).
LOVD TSC2 Database Review
The TSC1 and TSC2 Leiden Open Variation Databases (LOVD) provide a carefully curated listing of TSC1 and TSC2 variants identified in many different laboratories working on TSC (http://chromium.lovd.nl/LOVD2/TSC). In addition to the individuals discussed above, there are at least 11 individuals reported to have the TSC2 c.1864C>T variant in their germline (five probands and six relatives) in the TSC2 LOVD. No clinical details are available for two individuals. One patient has a definite diagnosis of TSC with no details provided. Another has a renal angiomyolipoma and an unspecified number of depigmented lesions which, depending on the number and size, may meet the criteria for a clinical diagnosis. Two individuals have an unspecified diagnosis of TSC, but no clinical details are provided. No other individuals in the LOVD meet clinical diagnostic criteria for definite TSC based on the phenotypic information provided. One has a diagnosis of possible TSC but no details are provided, another has a cortical tuber, and another has rhabdomyoma. Two are listed as asymptomatic but do not appear to have had a full work up (Suppl. Table SI).
DISCUSSION
There are relatively few specific genotype–phenotype correlations in TSC and individuals with the same genotype often have different manifestations and severity of disease. Thus, it is difficult to provide accurate prognostic information and compels all patients with TSC to undergo rigorous and costly surveillance. Clinicians have particular difficulty when patients are diagnosed in infancy or prenatally, prior to the appearance of many of the clinical signs, and it is likely that the number of patients diagnosed with TSC in infancy will increase. There are several reasons for this. First, prenatal ultrasound technology has improved, increasing the detection rate for rhabdomyoma. Second, genetic testing is readily available and recommended for rhabdomyoma due to the high association with TSC [Harding and Pagon, 1990]. Third, diagnostic criteria for TSC were revised in 2012 to include a pathogenic TSC1 or TSC2 variant as sufficient to diagnose TSC [Northrup and Krueger, 2013]. Therefore, it is important to identify pathogenic variants that may confer a restricted phenotype.
While, in general, specific genotypic data has not been able to predict phenotype, a few genotype–phenotype correlations have been identified. Overall, pathogenic TSC2 variants are associated with a more severe phenotype than pathogenic TSC1 variants [Dabora et al., 2001; Lewis et al., 2004; Sancak et al., 2005; Au et al., 2007; Kothare et al., 2014]. However, specific TSC2 variants have also been associated with milder phenotypes [Khare et al., 2001; O'Connor et al., 2003; Jansen et al., 2006; Wentink et al., 2012; van Eeghen et al., 2013; Ekong et al., 2016].
The TSC2 c.1864C>T, p.(Arg622Trp) variant in exon 17 is pathogenic according to the American College of Medical Genetics and Genomics (ACMGG) criteria (P3 and P4) [Richards et al., 2015] and probably pathogenic according to the TSC2 LOVD. Functional studies have demonstrated reduced expression, destabilization of the TSC protein complex, and increased activation of mTORC1 [Qin et al., 2010; Hoogeveen-Westerveld et al., 2011]. The variant has only been reported in families with features of TSC and is not found in large variant databases. For these reasons, we consider the TSC2 c.1864C>T, p.(Arg622Trp) variant to be pathogenic.
According to the 2012 consensus diagnostic criteria, the presence of a pathogenic variant is sufficient to diagnose TSC [Northrup and Krueger, 2013], thus, all the presented individuals meet diagnostic criteria for TSC. However, most of the individuals in our study did not meet the clinical criteria for definite TSC. Of the 13 patients with detailed phenotypic data, one met two major diagnostic criterion, ten met one, and two met no diagnostic criteria. In the patient with polycystic kidneys, the specific pathogenicity of the TSC2 variant for multiple cysts is difficult to estimate, given the presence of VUS in PKD1. The oldest variant-positive patient described here had only two renal cortical cysts by age 59 years. In addition to the patients for whom we have detailed phenotypes, at least five of the nine patients for whom there is clinical information in the TSC2 LOVD did not meet the clinical criteria for definite TSC. Only one individual is reported to have definite TSC, although no specific clinical information is available. In another patient it is not clear whether there were sufficient clinical criteria for definite TSC, while two other patients are reported to have TSC, but no other phenotypic information is provided. In summary, of the 22 patients with the TSC2 c.1864C>T variant for whom we have clinical information, at least 16 do not meet criteria for a clinical diagnosis of definite TSC. Prior to the 2012 consensus conference, these patients would not have been diagnosed with definite TSC.
Interestingly, all of the young children presenting with the variant (N = 7) had rhabdomyoma or signs of regressing rhabdomyoma, indicating a potential association. Rhabdomyomas are often asymptomatic and involute with time so the older reported individuals may have had them and were unaware. Another possibility is that patients with this variant and no rhabdomyoma would not present at clinic, and thus, would only be genotyped in familial cases. Cortical dysplasia was another common finding, reported in six of the presented patients.
Our analysis indicates that the TSC2 c.1864C>T p.(Arg622Trp) variant is consistently associated with a mild phenotype wherein manifestations associated with TSC are present but rarely clinically significant. This finding has major implications regarding prognosis and family planning. With the growing number of genotypes associated with mild phenotypes, clinicians should consider the use of genotyping as a prognostic tool. Moreover, as additional information is gathered, consideration should be given to altering surveillance recommendations for variants with mild phenotypic expression. Similarly, it will be important to assess whether pathogenic TSC1 and TSC2 variants should continue to be sufficient for a diagnosis of TSC, or whether there would be benefit to classifying a genetic diagnosis separately from a clinical diagnosis.
ACKNOWLEDGMENTS
LSF is the TSC Rare Disease Clinical Research Network (RDCRN) Fellow (1U54NS092090-01). The authors would also like to acknowledge funding support from the Autism Center of Excellence Network (1U01NS082320-01) and the RDCRN (1U54NS092090-01). WTG is supported by an Intramural Clinician Scientist salary award from BC Children's Hospital, and gratefully acknowledges the logistical support of K Townsend of the BCCH Biobank, who extracted and shipped DNA samples, and also performed Sanger validation of the familial TSC2 variant, for members of family 1 who live in BC. MN would like to acknowledge support from the Michelle Foundation and TS Association (UK).