Missense variant in UBA2 associated with aplasia cutis congenita, duane anomaly, hip dysplasia and other anomalies: A possible new disorder involving the SUMOylation pathway
Abstract
We report a patient with aplasia cutis congenita, Duane anomaly, hip dysplasia, and other anomalies who had a de novo missense variant in UBA2, which encodes for a protein involved in the SUMOylation pathway. It has previously been suggested that UBA2 haploinsufficiency underlies scalp defects in the 19q13.11 deletion syndrome. We propose that disturbance of the SUMOylation pathway, mediated by pathogenic variants in UBA2, is a novel mechanism for aplasia cutis congenita and other phenotypic abnormalities. © 2017 Wiley Periodicals, Inc.
INTRODUCTION
Aplasia cutis congenita (ACC) involving the scalp can occur in isolation or as a component of various well-defined syndromes. Nonsyndromic scalp defects have been described, including the five generation family reported by Marneros [2013] in which the cause was a heterozygous mutation in BMS1 (OMIM #107600). Mendelian syndromes with scalp defects include Johanson–Blizzard (OMIM #243800), scalp-ear-nipple (OMIM #181270), Adams–Oliver syndrome (AOS) (OMIM #100300), and others. In addition to Mendelian conditions, some chromosomal disorders are associated with scalp defects. Malan et al. [2009] delineated the 19q13.11 deletion syndrome, which includes scalp defects, microcephaly, developmental disabilities, hypospadias, growth retardation, and other anomalies. Melo et al. [2015] suggested that UBA2, which encodes ubiquitin-like modifier-activating enzyme 2, plays a role in the occurrence of scalp defects associated with this deletion syndrome.
In the current report, we describe a 2 years 6 months old female with scalp defects, Duane anomaly, and bilateral hip dysplasia. She had a tall forehead and mild bilateral fifth finger clinodactyly. Whole exome sequencing revealed a de novo, heterozygous missense variant in UBA2. This result reinforces the hypothesis that UBA2 plays a role in the scalp defects in the 19q13.11 deletion syndrome and suggests UBA2 as a novel candidate for Mendelian scalp defects and other anomalies.
CLINICAL REPORT
The patient was born at full term with a birthweight of 2,866 g (25th centile). There were no significant pregnancy complications. She was born with aplasia cutis congenita of the posterior scalp. During infancy, she was delayed in motor skills and at 18 months was evaluated for a hip click. Radiographs and CT scan of the pelvis showed bilateral femuroacetabular dysplasia, marked delay in the formation of the femoral ossific nuclei and bilateral hip dislocation. She underwent bilateral adductor tenotomy and closed surgical reduction for the hip dislocations. Review of systems was significant for Duane anomaly, strabismus, recurrent ear infections, croup, tonsillitis, and poor weight gain. Evaluation in gastroenterology clinic did not identify a cause for the poor weight gain. Renal ultrasound showed no abnormalities of the kidneys and bladder. Development and cognition were age appropriate at age two and half years. Review of the family history over three generations revealed no scalp defects, hip dysplasia, or genetic syndromes.
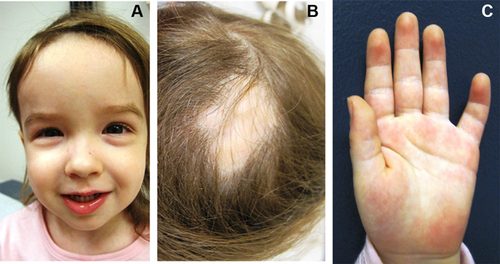
Physical exam at 2 years 6 months was significant for a weight of 10.7 kg (3rd centile). Her height was 88 cm (25th–50th centile), and her OFC was 48 cm (25th–50th centile). Her forehead appeared tall (Fig. 1). She had a healed area of scalp defect in the mid upper occipital area of the skull (Fig. 1). Her chest was normal, and her heart had a regular rate and rhythm. Her abdomen was soft with no organomegaly. She had bilateral mild fifth finger clinodactyly (Fig. 1), but no other limb anomalies. She had normal muscle tone.
METHODS
Informed consent was obtained from all individuals included in this report. In the case of minors, consent is obtained from one of the parents or legal guardians. Genomic DNA was extracted from whole blood from the affected child and both parents. Exome sequencing at GeneDx was performed on exon targets isolated by capture using the Agilent SureSelect Clinical Research Exome kit (Agilent Technologies, Santa Clara, CA). The sequencing methodology and variant interpretation protocol has been previously described [Tanaka et al., 2015]. The general assertion criteria for variant classification are publicly available on the GeneDx ClinVar submission page (http://www.ncbi.nlm.nih.gov/clinvar/submitters/26957/). The mean depth coverage was 148x, with 97.2% of the exome covered by at least 10 sequence reads.
DISCUSSION
There have been significant advances in the delineation of the molecular bases of aplasia cutis congenita. Although, genes associated with Adams–Oliver syndrome (ARHGAP31, DOCK6, RBPJ, EOGT, NOTCH1), scalp-ear-nipple syndrome (KCTD1), and familial ACC (BMS1) function in separate pathways, the characteristics of the skin defects in these conditions are indistinguishable, suggesting common pathogenetic mechanisms [Marneros, 2015]. The scalp is the most common site for ACC. Rapid skull expansion during prenatal brain development disproportionately increases the requirement for proliferation and differentiation of skin cells at this location, making it more vulnerable to genetic disturbances of skin morphogenesis [Marneros, 2015].
The patient we report had a de novo missense variant in UBA2 which encodes for ubiquitin-like modifier-enzyme 2. The variant is denoted c.71G>T and causes a conservative amino acid substitution p.Gly24Val (p.G24V). The p.G24V variant occurs at a position conserved across species and is predicted to be probably damaging to the protein structure/function by Provean, Mutation Taster, and CADD. The p.G24V variant was not seen in the NHLBI Exome Sequencing Project nor in the Exome Aggregation Consortium (ExAC) databases, indicating that it is not a common benign variant. Furthermore, there are no loss of function alleles in the ExAC database and missense variants are underrepresented, suggesting this highly conserved gene is intolerant to these changes. No other variants were reported in this patient.
Although, there are no known human disorders associated with mutations in UBA2, this gene is deleted in most patients with the 19q13.11 deletion syndrome. Venegas-Vega et al. [2014] reported a patient with this deletion and summarized the clinical features and deletion breakpoints of 11 cases from the literature. ACC was not observed in the one patient whose deletion did not involve UBA2 [Gana et al., 2012, (patient 2)]. Of the 11 patients whose deletion involved UBA2, 9 were mentioned to have ACC of the scalp. Of the other two, ACC was not present in one, while the presence or absence of ACC was not mentioned for the other. Subsequently, Melo et al. [2015] postulated a role for UBA2 in the occurrence of ACC in the 19q13.11 deletion syndrome.
Similarly, the fifth finger clinodactyly observed in our patient has been reported in the majority of patients with 19q13.11 deletion, but not in the patient whose deletion did not involve UBA2. As with our patient, high forehead has been reported in several patients with 19q13.11 deletion, but the presence or absence of this finding was not mentioned in the patient whose deletion did not include UBA2 [Gana et al., 2012 (patient 2)].
Hip dysplasia, which was observed in our patient, has been reported in four patients with the 19q13.11 deletion syndrome [Kulharya et al., 1998; Gana et al., 2012 (patient 1); Venegas-Vega et al., 2014; Melo et al., 2015] and has been reported in one such patient (patient 127) listed in the DECIPHER database [Firth et al., 2009].
Since Duane anomaly has not been reported in the 19q13.11 deletion syndrome, we specifically reviewed SALL1, SALL4, CHN1, HOXA1, and MFAB. No reportable variants were found in these genes. We did not find reports of Duane anomaly in patients with 19q13.11 deletion listed in the DECIPHER database [Firth et al., 2009].
The mechanism by which UBA2 mutations may cause scalp defects and other anomalies is unclear. It is unknown whether missense variants would have the same or different phenotypic effect as gene deletions. The gene product, ubiquitin-like modifier-activating enzyme 2, forms a heterodimer with SUMO1-activating enzyme 1 (SAE1). UBA2 plays a role in SUMOylation, a post-translational modification that adds small ubiquitin-like modifier (SUMO) proteins to lysine residues, regulating protein structure, intracellular localization, or function [Okuma et al., 1999; Venegas-Vega et al., 2014]. SUMOylation has been shown to regulate cell proliferation [Ding et al., 2016; Meng et al., 2016] and inactivation of the SAE1/UBA2 complex impairs the growth of NOTCH1 activated cells [Licciardello et al., 2015]. Defects in NOTCH1 are a common cause of AOS [Stittrich et al., 2014; Southgate et al., 2015].
We propose a potential mechanism for aplasia cutis congenita which involves abnormal SUMOylation mediated by defects in UBA2. Further reports of patients with UBA2 variants and functional studies of the variants are needed for further evaluation of this possibility. The ACC observed in our patient may be the sole phenotypic effect of the p.Gly24Val UBA2 variant. However, the presence of clinodactyly and high forehead in our patient may also be related to the UBA2 variant, as these features were reported in patients with 19q13.11 deletion syndrome, but were not found or not mentioned in the one reported patient whose deletion did not involve UBA2 [Gana et al., 2012]. Hip dislocation has been reported in five patients, whereas Duane anomaly has thus far not been reported in patients with the deletion. The lack of other variants on whole exome sequencing that could explain hip dysplasia and Duane anomaly suggest that these findings may also be causally related to the UBA2 variant. Thus, it is possible that pathogenic missense variants in UBA2 cause a novel genetic syndrome, the main features of which are aplasia cutis congenita, Duane anomaly, hip dysplasia, clinodactyly, and high forehead. Moreover, other than Duane anomaly, UBA2 haploinsufficiency may underlie these phenotypic features in the 19q13.11 deletion syndrome.
ACKNOWLEDGMENTS
We thank the parents for giving permission to publish clinical photographs.