Molecular and clinical analysis of ALPL in a cohort of patients with suspicion of Hypophosphatasia
Abstract
Hypophosphatasia (HPP) is a rare autosomal dominant or recessive metabolic disorder caused by mutations in the tissue nonspecific alkaline phosphatase gene (ALPL). To date, over 300 different mutations in ALPL have been identified. Disease severity is widely variable with severe forms usually manifesting during perinatal and/or infantile periods while mild forms are sometimes only diagnosed in adulthood or remain undiagnosed. Common clinical features of HPP are defects in bone and tooth mineralization along with the biochemical hallmark of decreased serum alkaline phosphatase activity. The incidence of severe HPP is approximately 1 in 300,000 in Europe and 1 in 100,000 in Canada. We present the clinical and molecular findings of 83 probands and 28 family members, referred for genetic analysis due to a clinical and biochemical suspicion of HPP. Patient referrals included those with isolated low alkaline phosphatase levels and without any additional clinical features, to those with a severe skeletal dysplasia. Thirty-six (43.3%) probands were found to have pathogenic ALPL mutations. Eleven previously unreported mutations were identified, thus adding to the ever increasing list of ALPL mutations. Seven of these eleven were inherited in an autosomal dominant manner while the remaining four were observed in the homozygous state. Thus, this study includes a large number of well-characterized patients with hypophosphatasemia which has permitted us to study the genotype:phenotype correlation. Accurate diagnosis of patients with a clinical suspicion of HPP is crucial as not only is the disease life-threatening but the patients may be offered bone targeted enzymatic replacement therapy. © 2017 Wiley Periodicals, Inc.
INTRODUCTION
Hypophosphatasia (HPP) is a rare genetic condition due to deficiency of the tissue nonspecific alkaline phosphatase (TNSALP), a member of the alkaline phosphatase (ALP) family of proteins. It is caused by mutations in ALPL, located on chromosome 1p36.12 [Millan and Whyte, 2015]. The ALP family includes TNSALP which is mainly expressed in bone, liver, and kidney and three tissue-specific proteins: intestinal (IAP), placental (PLAP-1), and placental-like (GCAP) proteins, encoded by three different genes: ALPI, ALPP, and ALPPL2, respectively [Kam et al., 1985; Berger et al., 1987; Millan and Manes, 1988]. TNSALP functions as an homodimer and has four different functional domains: (i) the catalytic site; (ii) the Ca2+ binding domain; (iii) the crown domain which is involved in modulation of the protein surface, oligomerization, domain–domain interaction, and stabilization, among others [Lee et al., 2014]; and (iv) the N-terminal alpha helix. The active isoform of TNSALP is a homodimer and its specific function is to cleave several phosphocompounds such as: inorganic pyrophosphate (PPi), pyridoxal-5-phosphate (PLP), and phosphoethanolamine (PEA) [Russell, 1965; Macfarlane et al., 1988; Macfarlane et al., 1991]. Thus, deficiency in TNSALP leads to the extracellular accumulation of these substrates, which results in defects in bone and teeth mineralization, among other clinical features. PPi acts by blocking the synthesis of hydroxyapatite crystals in the process of bone mineralization. PLP is the principal circulating form of vitamin B6 [Rockman-Greenberg, 2013]. Deficiency in TNSALP causes a decrease in the dephosphorylation of PLP to pyridoxal (PL), a compulsory step allowing pyridoxine to be transported across the blood–brain barrier. Increases in PLP in the systemic circulation indicates impaired transport of vitamin B6 to the brain, which leads to manifestation of seizures in the severe form of HPP [Mornet, 2008]. The role of PEA in the pathophysiology of HPP is yet to be fully understood.
HPP can be clinically subdivided into five groups: perinatal (MIM 241500), infantile (MIM 241500), childhood (MIM 241510), adult (MIM 146300), and odontohypophosphatasia (OHPP, MIM 146300) according to the age of onset [Rockman-Greenberg, 2013]. A lack of enzymatic activity may be detected in some patients while residual enzyme activity may be observed in others [Zurutuza et al., 1999; Moulin et al., 2009; Whyte et al., 2015b].
The incidence of the severe forms of this disease is estimated to be approximately 1 in 300,000 in European populations and 1 in 100,000 in Canadian population, probably due a founder effect in the Mennonite Canadian population [Fraser, 1957; Greenberg et al., 1993; Mornet et al., 2011; Leung et al., 2013] and very rare in black populations [Whyte, 2013]. The prevalence of the mild forms, those that do not compromise survival and whose symptoms appear in the childhood has been estimated at 1/6,000 [Mornet, 2015].
Currently, specific bone targeted recombinant enzyme replacement therapy (ERT) (Strensiq™-asfotase alfa) is available for HPP patients. This treatment has been shown to increase the survival rate, and improve the osteopenia and skeletal manifestations [Whyte et al., 2012, 2015a]. Some adverse effects have been described related to the study treatment of asfotase alfa which includes: reaction at the injection site (erythema), respiratory distress, craniosynostosis, and conductive hearing loss. Among these adverse effects, the authors suggested that the craniosynostosis was not related to asfotase alfa but due to a complication of the severe hypophosphatasia [Whyte et al., 2012].
In this manuscript, we report the results of a series of 83 probands and 28 relatives with a clinical and/or biochemical suspicion of HPP. Our first aim was to identify and characterize the molecular basis of patients with suspicion of HPP and second, to compare the clinical features in patients with and without ALPL mutations.
MATERIALS AND METHODS
Patients
The cohort included a total of 83 patients [age: 36 ± 22 years], 28 males and 55 females, and 28 family members, referred from 11 different hospitals from six countries: Spain (n = 45), Italy (n = 1), Russia (n = 24), Egypt (n = 4), United Arab Emirates (n = 7), and Denmark (n = 1) En tabla I aparece Portugal y Emiratos Arabes. Preliminary results of 39 of the adult patients were recently described [Riancho-Zarrabeitia et al., 2016]. This project was approved by the local ethical research board and all patients or parents gave informed consent to participate in this study.
We collected clinical, biochemical, and molecular data on all patients from their managing physician using a case report questionnaire with Human Phenotype Ontology (HPO) classification (Supplementary Table SI) [Kohler et al., 2014]. The degree of severity was determined by clinical assessment and their age of onset (Supplementary Table SI) and patient classification was undertaken according to the age of the first clinical manifestation. Severe HPP has been classified as patients with perinatal onset detected by fetal sonography and include all or a combination of the following clinical features: multiple prenatal fractures, apnea, respiratory failure, delayed closure of anterior fontanel, abnormality of long bone morphology and/or bone mineralization, and may result in stillbirth or death shortly after [Millan and Whyte, 2015]. Moderate HPP was usually assigned to those patients with clinical features in the infantile and childhood stages, and include: short stature, recurrent fractures, muscle weakness, myalgia, bone pain, low alkaline phosphatase, generalized bone demineralization, developmental regression, hypercalciuria, and infantile hypercalcemia. Finally, those patients with clinical manifestations in adulthood were classified as less severe HPP, with low alkaline phosphatase, bone pain, muscle weakness, osteopenia, myalgia, premature loss of teeth, generalized bone demineralization, hypotonia, fracture of long bones, and recurrent fractures. However, some children without skeletal abnormalities were classified as OHPP.
Mutation Analysis
Oligonucleotides were designed for all coding exons and exon-intron boundaries of the ALPL transcript NM:_000478.4, with the help of Primer v3 Software [Koressaar and Remm, 2007] and SNPCheck V3 [Koressaar and Remm, 2007] (Supplementary Table SII). All amplicons were sequenced (BrightDye, Nimagen, The Netherlands) on an ABI3730XL DNA analyzer (Life Technologies).
To assess the possible pathogenicity of the variants, we applied several in silico tools: MutationTaster, Polyphen2, CADD V1.3 [Kircher et al., 2014], MutPred and splicing programs present in Alamut V2.7.2. We additionally searched the ALPL gene mutation database curated by Prof. Mornet, (http://www.sesep.uvsq.fr/03_hypo_mutations.php) as well as the Human Gene Mutation Database (HGMD, Cardiff University, UK). In addition, we determined the frequencies of the putative pathogenic variants in control populations (Exome Aggregation Consortium, ExAc) (http://exac.broadinstitute.org/), 1000G (www.1000genomes.org), and Exome Variant Server (EVS) (http://evs.gs.washington.edu/EVS/). Additionally, we applied the ACMG guidelines to classify the variants according their criteria's [Richards et al., 2015].
RESULTS
A total of 30 different ALPL mutations were found in 36 of the 83 probands (Table I). In silico pathogenicity prediction and population frequencies are shown for all novel mutations (Table II). The distribution of the mutations represented across the gene, type of variant, and HPP form are listed in Figure 1. Eight mutations were shown to co-segregate with the phenotype in 28 related members from seven families (Supplementary Table SIV)
| Patient ID | HPP form | cDNA | Amino acid | Country of origin | Citation | |
|---|---|---|---|---|---|---|
| 1 | HPP1 | Adult | c.334G>C | p.Gly112Arg | Spain | Witters et al. [2004] |
| 2 | HPP2 | Infantile | c.[571G>A];[1133A>G] | p.[Glu191Lys];[(Asp378Gly)] | Portugal | Henthorn et al. [1992] |
| This study | ||||||
| 3 | HPP4 | Adult | c.334G>A | p.Gly112Ser | Spain | Witters et al. [2004] |
| 4 | HPP5 | Adult | c.382G>A | p.Val128Met | Spain | Mumm et al. [2002] |
| 5 | HPP7 | Adult | c.1366G>A | p.Gly456Arg | Spain | Ozono et al. [1996] |
| 6 | HPP10 | Adult | c.497C>T | p.(Thr166Ile) | Spain | This study |
| 7 | HPP15 | Adult | c.334G>C | p.(Gly112Arg) | Spain | This study |
| 8 | HPP18 | Adult | c.542C>T | p.Ser181Leu | Spain | Lia-Baldini et al. [2001] |
| 9 | HPP19 | Adult | c.443C>T | p.Thr148Ile | Spain | Spentchian et al. [2003] |
| 10 | HPP21 | Adult | c.443C>T | p.Thr148Ile | Spain | Spentchian et al. [2003] |
| 11 | HPP23 | Adult | c.1417G>A | p.Gly473Ser | Spain | Mornet et al. [1998] |
| 12 | HPP24 | Adult | c.1083_1084dupGCAG | p.(Ser364Argfs*42) | Spain | This study |
| 13 | HPP26 | Adult | c.473-2A>G | N/A | Spain | This study |
| 14 | HPP28 | Adult | c.454C>T | p.Arg152Cys | Spain | Versailles lab Nov. 2009, unpublished |
| 15 | HPP29 | Adult | c.407G>A | p.Arg136His | Spain | Taillandier et al. [1999] |
| 16 | HPP31 | Adult | c.497C>T | p.(Thr166Ile) | Spain | This study |
| 17 | HPP33 | Adult | c.358G>A | p.Gly120Arg | Spain | Mornet et al. [1998] |
| 18 | HPP34 | Adult | c.542C>T | p.Ser181Leu | Spain | Lia-Baldini et al. [2001] |
| 19 | HPP35 | Adult | c.[352C>A]; [c.352C>A] | p.(Leu118Met);(Leu118Met) | Spain | This study |
| 20 | HPP36 | Adult | c.871G>A | p.Glu291Lys | Spain | Mornet et al. [1998] |
| 21 | HPP37 | Adult | c.383_384insG | p.(Val130Glyfs*6) | Spain | This study |
| 22 | HPP40 | Adult | c.512A>G | p.His171Arg | Spain | [Mornet., unpublished] |
| 23 | HPP41 | Adult | c.497C>T | p.(Thr166Ile) | Spain | This study |
| 24 | HPP48* HPP49* | Perinatal | c.[815G>T];[874C>T] | p.[(Arg272Leu)];[(Pro292Ser)] | Egypt | Spentchian et al. [2003] |
| This study | ||||||
| 25 | HPP50^ | Perinatal | c.[815G>T]; [874C>T] | p.[(Arg272Leu)];[(Pro292Ser)] | Egypt | Spentchian et al. [2003] |
| This study | ||||||
| 26 | HPP64 | Adult | c.436G>A | p.Glu146Lys | Russia | This study |
| 27 | HPP66 | Childhood | c.61G>A | p.(Glu21Lys) | Russia | This study |
| 28 | HPP71 | Adult | c.984_986delCTT | p.Phe328del | Russia | Orimo et al. [1997] |
| 29 | HPP74 | Adult | c.571G>A | p.Glu191Lys | Russia | Herasse et al. [2002] |
| 30 | HPP78 | Childhood | c.659G>T | p.Gly220Val | Russia | Taillandier et al. [2001] |
| 31 | HPP90 | Childhood | c.211C>G | p.(Arg71Gly) | Russia | This study |
| 32 | HPP94 | Childhood | c.340G>A | p.Ala114Thr | Denmark | Mumm et al. [2001] |
| 33 | HPP95 | Childhood | c.343_348dupACCGCC | p.Thr115_Ala116dup | Spain | Versailles lab March 2009, unpublished |
| 34 | HPP104 | Infantile | c.[407G>A];[c.1540G>A] | p.[(Arg136His)];[(Ala514Thr)] | United Arab Emirates | Taillandier et al. [1999] |
| This study | ||||||
| 35 | HPP109 | Infantile | c.1064T>C;c.1064T>C | p.Met355Thr | Egypt | Versailles lab March 2009, unpublished |
| 36 | HPP170 | Infantile | c.567_568insT | p.Asn190* | Spain (French ancestry) | This study |
- Patients From HPP4 to HPP41 Have Been Recently Published [Riancho-Zarrabeitia et al., 2016]
- *Affected twins and ^his brother.
| “In silico analysis” | Control population frequency analysis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proband | HPP severity group | Inheritance pattern | Exon | cDNA position | Protein position | Genomic location (GRCh38/hg38) | CADD V1.3 | Polyphen 2 | MutationTaster | MutPred | SNP&Go | ExAc | 1,000 G | EVS (n = 6,500) | Present in ALPL database (Mornet) | ||
| HPP66 | Childhood | AD | 2 | c.61G>A | p.(Glu21Lys) | chr1:21,880,635 | 24.2 | Possibly damaging (0.848) | DC 1 | 0.386 | Neutral = 2 | Absent | Absent | Absent | No | ||
| HPP90 | Childhood | AD | 4 | c.211C>G | p.(Arg71Gly) | chr1:21,887,619 | 33 | Probably damaging (1) | DC 0.999 | 0.976 | Disease = 5 | Absent | Absent | Absent | No | ||
| HPP35 | Adult | AR | 5 | c.352C>A | p.(Leu118Met) | chr1:21,563,164 | 27 | Probably damaging (1) | DC 0.999 | 0.577 | Neutral = 7 | Absent | Absent | Absent | No | ||
| HPP37 | Adult/OHPP | AD | 5 | c.383_384insG | p.(Val130Glyfs*6) | chr1:21,563,196 | 35 | N/A | DC 1 | N/A | N/A | Absent | Absent | Absent | No | ||
| HPP10;HPP31;HPP41 | Adult/OHPP | AD | 6 | c.497C>T | p.(Thr166Ile) | chr1:21,564,065 | 32 | Probably damaging (1) | DC 0.999 | 0.797 | Disease = 4 | Absent | Absent | Absent | No | ||
| HPP170 | Childhood | AD | 6 | c.567_568insT | p.(Asn190*) | chr1:21,890,628_21,890,629 | 34 | N/A | DC 1 | N/A | N/A | Absent | Absent | Absent | No | ||
| HPP48;HPP49 | Perinatal | AR (CH) | 9 | c.874C>T | p.(Pro292Ser) | chr1:21,900,169 | 25.3 | Probably damaging (0.999) | DC 0.999 | 0.515 | Disease = 4 | Absent | Absent | Absent | No | ||
| HPP2 | Infantile | AR (CH) | 10 | c.1133A>G | p.(Gln378Gly) | chr1:21902361 | 28.6 | Probably damaging (1) | DC 0.999 | − | Disease = 8 | Absent | Absent | Absent | No | ||
| HPP24 | Adult | AD | 10 | c.1091_1092insGCAG | p.(Ser364Argfs*42) | chr1:21,575,823 | 23.8 | N/A | DC 1 | N/A | N/A | Absent | Absent | Absent | No | ||
| HPP26 | Adult/OHPP | AD | IVS5 | c.473-2A>G | N/A | chr1:21,564,039 | 24.8 | N/A | DC 1 | N/A | N/A | Absent | Absent | Absent | No | ||
| HPP109 | Infantile | AR | 10 | c.1064T>C | p.(Met355Thr) | chr1:21,902,292 | 24 | Probably damaging (0.958) | DC 0.999 | N/A | Disease = 1 | Absent | Absent | Absent | Yes* | ||
- N/A, Not applicable; Inheritance Pattern; AD, Autosomal dominant; AR, Autosomal recessive; CH, Compound heterozygous.
- Silico pathogenicity prediction analyses and frequencies in control populations. CADD V1.3: CADD is a tool for scoring the deleteriousness of single nucleotide variants as well as insertion/deletions variants in the human genome. CADD can quantitatively prioritize functional, deleterious, and disease causal variants across a wide range of functional categories, effect sizes, and genetic architectures and can be used prioritize causal variation in both research and clinical settings. We adopted the following ranges of the Phred score: [0–10] probably benign; [10–14] unknown; [>14] probably pathogenic. MutationTaster: (score ranges from 0 to 1; values close to 1 indicate pathogenicity “DC = Disease Causing”). Polyphen 2: calculates the percentage in which the mutation can be damaging. MutPred (g significance range from 0 to 1, indicating the probability of a deleterious mutation. The high score of g, the highest probability of deleterious mutation). All variants are absent in the analyzed control populations (ExAC. data shown for 1000G (1000 genomes) and 6500 exomes at Exome Variant Server (EVS)).
- * This variant is present in an autosomal dominant fashion in the Mornet database.
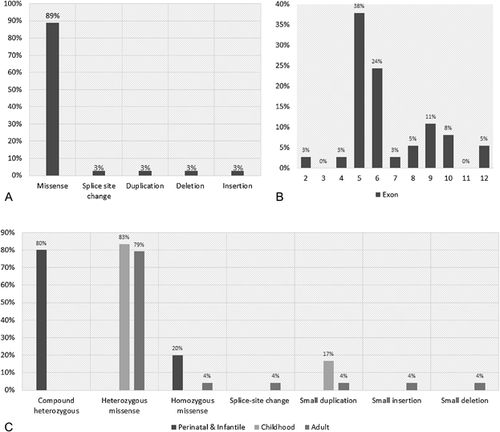
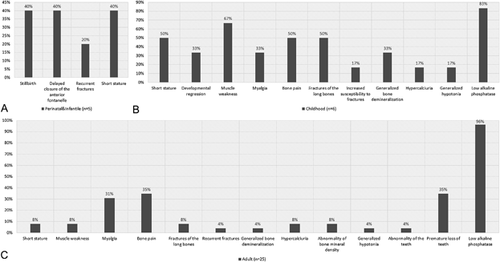
The frequency of the clinical manifestations for each HPP form are shown in Figure 2 and detailed information about HPO terms associated to each mutation (dominants [heterozygous] and recessives [both homozygous and compound heterozygous]) are shown in Figure 1. Detailed clinical phenotype of the mutation positive probands is shown in Supplementary Table SIII, according to the HPP groups, perinatal and infantile, childhood and adult.
DISCUSSION
We clinically and molecularly studied 83 probands with clinical and/or biochemical features suggestive of HPP, and 28 relatives (Supplementary Table SIV). Mutations were identified in 36 individuals (43.3%): six with clinically severe forms (three perinatal and three infantile), six childhood, and 24 adult forms of HPP (Supplementary Fig. S1). Co-segregation of the genotype was confirmed in seven families. The mutation rate was higher in females (69.4%) despite the gender distribution being similar (45% female, 55% male). This value is slightly higher than previously reported [Berkseth et al., 2013].
Eleven novel ALPL variants were identified, all of which are absent from large population databases (ExAc, 1,000 genomes and EVS) and were predicted to be pathogenic, strongly reinforcing that these mutations had a deleterious effect on the protein (Table II). Although some predictors provided a neutral effect of some variants, the absence of the variant in the control population databases, and the pathogenic estimation for the rest of the in silico predictors suggests that they may be pathogenic. Despite this, functional analysis, including enzyme activity determination, is required to clarify and quantify their pathogenicity. Seven of the undescribed variants followed an autosomal dominant pattern of inheritance whereas four were recessive. The variant p.Thr166Ile was detected in three unrelated patients of Spanish origin (Table I, HPP10, HPP31, and HPP41), suggesting that they share a common Iberian ancestor. Founder ALPL mutations have been previously reported in several populations [Greenberg et al., 1993; Herasse et al., 2002; Michigami et al., 2005].
The mutation p.Arg71Gly has not been previously described in the literature, although four different variants in the same amino acid position (additionally to the p.Arg71Gly) has been reported by three different groups (p.Arg71Ser, p.Arg71His, p.Arg71Pro, and p.Arg71Cys) [Henthorn et al., 1992; Orimo et al., 2001; Taillandier et al., 2001]. This fact could mean that the Arginine at position 71 of the protein seems to be a hot spot position for mutation due high mutation rate, as five different mutations have been reported at this amino acid. Additionally, this position is extremely high conserved through the evolution as it is conserved until D. melanogaster orthologue gene (cg8147), adding an evidence of the importance of this amino acid in the function of the wild-type alkaline phosphatase enzyme. The surrounding region near to the Arginine at position 71 is also conserved, which makes it an important clue to understand what important is this region within the domain of the protein.
We identified a compound heterozygous ALPL mutation, p.[(Arg272Leu)];[(Pro292Ser)] in three siblings from the same consanguineous family with perinatal HPP (HPP48, HPP49, and HPP50). Both twins died shortly after birth due to respiratory failure as the consequence of a several multifactorial defects. The other sibling had a severe postnatal form of HPP, characterized by a bone lesion near her left wrist that seemed to be an intrauterine healed fracture.
In another family, we detected an affected 5-year-old girl (HPP109) with a homozygous c.1064T>C (p.Met355Thr) mutation, who had delayed closure of the anterior fontanel, moderate to severe osteopenia, short stature, delayed tooth eruption, recurrent healed fractures formation since the age of three, bowed long bones, and cupping of the metaphyses. She was born to a consanguineous couple, who also had another child with severe HPP (DNA unavailable). Interestingly, this is the first time that this variant is described in homozygosity. Previous studies reported this variant in compound heterozygosity, in combination with another mutation, p.Gly221Val. The parents of the proband, carriers of this mutation in a heterozygous state, did not manifest any HPP associated clinical features.
Infantile HPP was assessed in patients that had their first clinical episode related to HPP after birth and before the age of 6 months. In general, the pattern of inheritance is autosomal recessive [Rockman-Greenberg, 2013; Whyte, 2013] as in the perinatal group but, in our cohort, we detected one proband with an autosomal dominant pattern (HPP104). This patient presents another variant, p.Ala514Thr, which have been predicted as a VOUS (“variant of unknown significance”) but in silico analyses do not predict it as having any pathogenic effect. This patient showed bone pain, muscle weakness, and infantile hypercalcemia probably due to low alkaline phosphatase levels.
As previous authors suggested [Taillandier et al., 2015; Whyte et al., 2015b], autosomal dominant mutations in ALPL are usually related to childhood, OHPP and adult HPP, because the other normal allele provides sufficient residual enzyme function, thus reducing the severity of the skeletal manifestations. But, no significant differences (with statistical P-value power) in the clinical severity were observed between probands with AD or AR inheritance, probably due to the limited cohort size, although it seems to be a tendency of more severe phenotype for AR patients.
Childhood HPP is classified when the first clinical manifestation appears between 6 months and 18 years of age. It is mainly characterized by rickets, muscle weakness, bone pain, poor physical performance, tooth anomalies (hypoplasia, premature loss of deciduous teeth, caries), and the biochemical profile includes low serum ALP activity (mandatory) and increased serum PPi, PLP, and urine PEA. Childhood HPP can be inherited in an autosomal recessive or dominant pattern [Henthorn et al., 1992; Zurutuza et al., 1999; Hu et al., 2000; Lia-Baldini et al., 2001]. In our series, we have identified mutations in six individuals with childhood HPP, five heterozygous missense mutations, and one patient with a duplication of six nucleotides (HPP95) (c.343_348dupACCGCC; p.Thr115_Ala116dup) described previously in patients with childhood HPP. Clinical features in at least 50% of patients with childhood HPP were: short stature, muscle weakness, bone pain, and fractures of long bones. ALP measurements were not provided in 17% of the childhood HPP group, therefore, diagnostic classification was only based on clinical features.
Adult HPP is given to individuals in whom clinical manifestations appeared after 18 years of age. Despite this classification, many adults stated that they had HPP symptoms during childhood, but were undiagnosed until later in life.
Patients exclusively showing dental impairment were referred as OHPP. Patients with this form of HPP may have either autosomal recessive or dominant modes of inheritance [Mornet, 2007]. In our adult OHPP group, 9/24 (37.5%) had ALPL mutations; seven had heterozygous missense mutations, one had a heterozygous canonical splice-site mutation (c.473-2A>G, HPP26), and one patient had a frameshift mutation. Three novel mutations were identified in this group (p.Val130Glyfs*6, p.Thr166Ile, and c.473-2A>G). The p.Thr166Ile was present in three unrelated individuals with OHPP. Additional findings included myalgia in one proband. The p.Val130Glyfs*6 mutation is predicted to result in a prematurely truncated protein which may be exposed to nonsense mediated decay (NMD). This patient presented with long bone fractures, an uncommon feature for adult HPP.
There were important differences in the clinical HPP presentations. In our series, perinatal and infantile HPP showed the clinical features described in Figure 2. It is important to remark that infantile and perinatal HPP cases were detected due to a familial history of HPP or severe skeletal defects during prenatal and/or perinatal stages. In childhood HPP, frequent features were (>50% of the patients): low ALP (83%), muscle weakness (67%), bone pain, long bone fractures, and short stature (50%) (Table II). In the adult HPP, the most common feature observed was low ALP levels, but a significant proportion also manifested with premature loss of teeth (35%), a specific feature for OHPP. The principal characteristics of childhood and adult HPP referrals were low ALP with mild bone manifestations.
Forty-seven probands (56.62%) were negative after ALPL screening. It is possible that some of these patients may have a differential diagnosis with another skeletal dysplasia such as osteogenesis imperfecta (OI), campomelic dysplasia, hypophostaemic rickets, cleidocranial dysplasia, or osteoporosis. One of the negative patients was stillborn and showed bowing of limbs due to multiple prenatal fractures and abnormalities of bone mineral density, all clinical features compatible with OI. Two other patients manifested isolated clinical features during the perinatal stage (apnea and multiple prenatal fractures), without low levels of ALP. On the other hand, patients with less severe clinical features, such as bone pain and muscle weakness, may have these features secondary to a primary injury and/or have a behavioral effect. The remaining negative patients showed clinical features during childhood (n = 16) or in adulthood (n = 28). This study has not screened for deletions, which are found in approximately 1.3% of all HPP forms [Mornet, 2008]. However, many of our negative patients had heterozygous SNPs in several exons or introns, which reduces the possibility of having large deletions.
Although there is an enzyme replacement therapy for HPP patients, none of the patients included in this study have received this, to date.
CONCLUSIONS
In summary, the identification of 11 novel ALPL mutations in the five different HPP forms and the observation of a recurrent mutation, p. (Thr166Ile) in the Spanish population expand our knowledge of pathogenic ALPL mutations. We also contribute to the understanding of this rare disease with a well-characterized population, which will help with the clinical management and follow-up. These data will be useful not only for geneticists, but also for pediatricians, pediatric endocrinologists, radiologists, dentists, and physicians who manage HPP patients.
ACKNOWLEDGMENTS
We want to thank all clinicians and families who were involved in the development of this project. This work was supported in part by the following grants: Comunidad de Madrid (ENDOSCREEN: S2010/BMD-2396), MINECO (SAF2012-30871; SAF2015-66831-R), Egyptian grant STDF 5253, and by an Alexion Pharmaceuticals grant.