Importance of a specialty clinic for individuals with fragile X syndrome
Abstract
Advances in human genetics have identified a significant number of genetic disorders associated with intellectual disability. As a result, appropriate clinical management of these affected individuals and their family members have become critical in addressing medical needs to improve quality of life. We examine the importance of a Fragile X Clinic for individuals with fragile X syndrome (FXS) and their family members by conducting a retrospective chart review of 123 new patients with FXS evaluated at the Fragile X Clinic at Emory University. After the initial diagnosis of a proband with FXS with cascade testing, there were 345 family members identified with a mutation (70% with premutations; 30% with full mutations). In terms of the impact of the clinic visit, males had a substantial number of new diagnoses in all behavioral disorders (P < 0.001), with anxiety (62%) being the most common. For female probands, the most frequent diagnosis was also anxiety (87%). Prior to the clinic visit, very few patients were prescribed psychotropic medications. After the clinic visit, the most frequently prescribed psychotropic medications for males were stimulants (41%; P < 0.001) and SSRIs (40%; P < 0.001). For females, only stimulants (33%; P = 0.03) and SSRIs (44%; P = 0.008) were statistically significantly prescribed. Our results revealed that there is a gap in care to address the co-morbid behavioral issues, psychopharmacologic medication management, and genetic counseling needs regarding FXS. A multidisciplinary setting and approach, such as that offered by a Fragile X Clinic, is one method of treating the complex needs of patients with FXS. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Advances in human genetics have identified a significant number of genetic disorders associated with intellectual and developmental disabilities, including autism spectrum disorder (ASD). Fragile X syndrome (FXS), the most common inherited form of intellectual disability, affects 1 in 4,000 males and 1 in 8,000 females and is associated with a CGG repeat expansion of over 200 repeats in the fragile X mental retardation 1 gene (FMR1) [Sherman, 2002]. The prevalence of premutation carriers (55–200 repeats) is about 1 in 1,200 females and 1 in 450 males [Tassone et al., 2012]. These individuals do not have FXS but are at risk for having children (females) or grandchildren (males and females) with FXS, and are at risk for fragile X-associated tremor/ataxia syndrome (FXTAS) and fragile X-associated primary ovarian insufficiency (FXPOI). Males with FXS have moderate-to-severe intellectual disability and females range from normal to moderate impairment [Hagerman et al., 2010]. Individuals with FXS are at increased risk for a range of co-occurring behavioral problems that may cause significant limitations in their academic, adaptive, daily living function, and social interactions [Hagerman et al., 2009]; approximately 40–50% of individuals with FXS are also diagnosed with ASD [Hall et al., 2008; Harris et al., 2008]. The most common behavioral conditions include attention problems, hyperactivity, anxiety, aggression, poor sleep, and self-injury. Additionally, individuals with FXS are prone to a variety of comorbid medical issues including seizures, recurrent otitis media, strabismus, gastrointestinal disturbances, and connective tissue problems [Kidd et al., 2014].
The diagnosis of FXS is important not just for the affected child, but also to the siblings, parents, and immediate and extended family members in each generation. The diagnosis of FXS often changes the lives of many families who suddenly encounter the challenges of understanding the genetics, medical, developmental, and psychiatric issues related to FXS. Parents may be overwhelmed by the unexpected diagnosis and as they struggle to understand what the future holds for their family, they are also expected to coordinate the necessary services for their child. Families of children with FXS experience a wide range of challenges, including the need for medical specialists, high caregiver demands, job consequences, and caregiver health and mental health issues [Bailey et al., 2012]. In addition to dealing with their child's needs, parents also have to inform other family members that their child has received the diagnosis of FXS and relay the potential implications for learning and behavioral problems associated with FXS and reproductive planning and the health of older family members. For these reasons, a Fragile X Clinic plays an important role in providing specialized evaluation and treatment recommendations to help the child as well as immediate and extended family members. By attending a Fragile X Clinic, families have access to coordinated and comprehensive care focusing not only on the child with FXS but other family members from medical specialists who are experts in fragile X-associated disorders (FXD) which include FXTAS and FXPOI.
In response to the community's needs for specialized medical care for their child with FXS and their families, the National Fragile X Foundation, a national support and education non-profit organization, and several of the existing Fragile X Clinics in the United States, established the Fragile X Clinical and Research Consortium (FXCRC) in 2006 [Sherman et al., in press, Pediatrics; Liu et al., 2016, companion paper, this issue]. The Fragile X Clinic at Emory University in Atlanta, GA was one of the founding clinics in the FXCRC, established in 2005 as part of the Division of Medical Genetics. At this clinic, patients are evaluated by clinicians with expertise in FXS (developmental-behavioral pediatrician, medical geneticist, educational psychologist, genetic counselor), and may be referred to occupational therapy, speech and language therapy, and behavioral therapy within the institution. Children with FXS are usually evaluated for behavioral problems and treatments may consist of behavioral therapy and/or psychopharmacology. The clinic also provides services and/or referrals for FXTAS and FXPOI with an adult neurologist or reproductive endocrinologist, respectively.
In order to gain an understanding of the impact of a specialty clinic, such as a Fragile X Clinic, on the care of individuals with FXS and their families, we reviewed the new diagnoses and pharmacological treatment recommendations that we provided as a result of the patient's first visit to our clinic. Additionally, clinical information gathered during the clinic visit will enhance our understanding of the condition as there is considerable variability in the medical features and behavioral phenotype, which has treatment implications. We also explore the unmet needs of children with FXS, and in a companion paper discuss the implications of the provision of high quality clinical care, research, and training at specialty clinics via the FXCRC.
MATERIALS AND METHODS
After receiving approval from the Emory University institutional review board, we conducted a retrospective chart review to identify new patients with FXS seen consecutively at the Fragile X Clinic at Emory University from 2005 to 2013. We abstracted data from the 123 new patients’ medical records utilizing the parent intake form, clinic medical record, and genetic counseling and pedigree reports. We collected information regarding the parents’ concerns and child's co-occurring diagnoses and treatment prior to the clinic visit, and then reviewed the child's new diagnoses and treatment recommendations after the clinic visit.
Newly diagnosed patients with FXS also received genetic counseling and cascade genetic testing as part of their clinic visit. Pedigree construction and analysis was conducted in a standardized manner using a cascade method [McConkie-Rosell et al., 2007; Finucane et al., 2012]. Through these pedigrees, the number of additional family members diagnosed with premutations or full mutations as confirmed by FMR1 DNA testing was determined after the initial diagnosis of each proband. An anonymous summary sheet was developed to standardize the pedigree review procedures and to track the number and gender of affected probands and family members.
Continuous data were analyzed using the mean, standard deviation, and range for the continuous variables, McNemar's chi-square test (paired or before-after comparisons), and the chi-square test for association for the comparisons by gender. Data analysis was performed using SAS, version 9.1 software (Cary, NC), and Epi-Info 7 (Centers for Disease and Prevention, Atlanta, GA).
RESULTS
Medical records were reviewed on a total of 123 (115 males and 18 females) patients (all new) with FXS with a mean age of 6.6 years (range = 5 months to 17 years). Race/ethnicity was described as follows: 76% Caucasian, 21% African American, 1% Hispanic, and 2% Asian. In our cohort, 100 (81%) were diagnosed based on neurodevelopmental concerns (e.g., speech delay) and 23 (19%) received FXS testing based on having a family history of FXS.
Only patients presenting with neurodevelopmental concerns (N = 100) were included in the analysis regarding age of diagnosis. Those with a family history were excluded given that they would have an earlier diagnostic trajectory not based on presentation of symptoms. Table I describes the age at first parental concern and at diagnosis of FXS. The mean age at both first parental concern and diagnosis was earlier for males than females, although not statistically significant, and the number of female patients was small. The average difference in years or lag time between first parental concerns and a FXS diagnosis was 27.8 months for males and 27 months for females.
| Mean age ± standard deviation (range) in months at 1st parental concern | Mean age ± standard deviation (range) in months at FXS diagnosis | |
|---|---|---|
| Males (N = 90) | 12.4 ± 8.5 (1.0 to 20.4) | 40.2 ± 26.0 (1.0 to 186.0) |
| Females (N = 10) | 16.0 ± 14.4 (12.0 to 36.0) | 43.0 ± 10.8 (28.8 to 49.2) |
| P-value for difference by gendera | P > 0.46 | P > 0.53 |
- a Comparing males to females within each column.
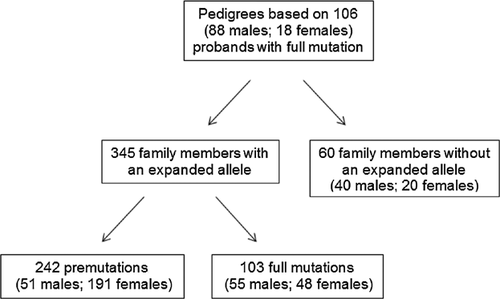
We were able to obtain a complete family pedigree constructed by our clinic's genetic counselor for 106 (18 females and 88 males) patients from our original cohort (see Fig. 1). Overall, there were 242 family members identified with a premutation or considered permutation carriers (70%; 51 males and 191 females), and 103 with a full mutation or FXS (30%; 55 males and 48 females). Thus, after the initial diagnosis of a proband with FXS and as a result of cascade testing, a substantial number of additional family members will be diagnosed with FXS or as premutation carriers, especially females as premutation carriers.
Prior to the Fragile X Clinic visit, male patients with FXS had the following past medical conditions diagnosed by their primary care doctor and/or specialists: 43% with chronic otitis media, 7% with seizures, 15% status post adenoidectomy or adenotonsillectomy, 2% with obstructive sleep apnea, and 3% with gastroesophageal reflux. Females with FXS presented a history of the following co-occurring medical conditions: 28% with chronic otitis media and 11% with status post adenoidectomy or adenotonsillectomy. No new medical issues were diagnosed for males or females after the visit to our Fragile X Clinic.
In terms of developmental and behavioral diagnoses (Table II), for each behavior or comorbid problem, less than 10% of males received any of the diagnoses prior to the clinic visit. For females, the most frequent diagnosis was anxiety (11%) prior to the clinic visit. As a result of the clinic visit, 70% of our patients were diagnosed with behavioral problems. For both males and females, anxiety was the most common post-clinic diagnosis, followed by sensory integration disorder. There was a statistically significant increase in the frequency of behavioral and comorbid diagnoses post-clinic visit (Table II). Notably, males had a substantial number of new diagnoses in all behavioral disorders (P < 0.001), whereas females had a notable increase in diagnoses of anxiety (P = 0.001) and sensory integration disorders only (P = 0.008). In comparisons by gender, females had statistically significantly more diagnoses of anxiety (P = 0.05) compared to males, and males had statistically significantly more diagnoses of aggression (P = 0.01) compared to females.
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Diagnosis of behavioral and comorbid problems | Prior to clinic visit (N = 115) | After clinic visit (N = variablea) | Before-after clinic visit comparison, P-valueb | Prior to clinic visit (N = 18) | After clinic visit (N = variablea) | Before-after clinic visit comparison, P-valueb | After clinic visit comparison by gender, P-valuec |
| ADHD | 11 (9%) | 53 (51%) | P < 0.001 | 1 (5%) | 5 (29%) | P = 0.06d | P = 0.12 |
| Anxiety | 6 (5%) | 68 (62%) | P < 0.001 | 2 (11%) | 14 (87%) | P = 0.001d | P = 0.05 |
| Aggression | 5 (4%) | 28 (25%) | P < 0.001 | 0 | 0 | NC | P = 0.01 |
| Sensory integration disorder | 7 (6%) | 61 (56%) | P < 0.001 | 0 | 8 (44%) | P = 0.008d | P = 0.44 |
| Autism | 9 (7%) | 32 (30%) | P < 0.001 | 1 (5%) | 2 (12%) | P = 0.50d | P = 0.15 |
- NC, not calculated (no diagnoses in particular group).
- a Number is variable depending on number diagnosed prior to clinic visit; all children diagnosed prior to clinic visit continued to be classified as such.
- b Based on McNemar chi-squared test for paired data.
- c Fisher's exact test.
- d Number of discordant pairs is less than 20, so exact results were used.
Prior to their clinic appointment, only a small proportion of the males and none of the females with FXS were prescribed any psychotropic medications related to their behavior problems (Table III). Among males, the most frequently prescribed psychotropic medications after the clinic visit were stimulants (41%) and SSRIs (40%), with a statistically significant increase in prescriptions of all psychotropic medication classes specified (P < 0.001 for stimulants and SSRIs; P = 0.002 for anti-psychotics and alpha agonists). For females, only stimulants (33%; P = 0.03) and SSRIs (44%; P = 0.008) were statistically significantly prescribed after the clinic visit (Table III). Overall, 60% of patients were prescribed psychotropic medications post-clinic visit. There were no statistically significant differences in prescriptions of psychotropic medications by gender. These particular analyses may have lacked statistical power due to the small numbers of females available for analysis.
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Prescribed psychotropic medications | Prior to clinic visit (N = 115) | After clinic visit (N = variablea) | Before-after clinic visit comparison, P-valueb | Prior to clinic visit (N = 18) | After clinic visit (N = variablea) | Before-after clinic visit comparison, P-valueb | After clinic visit comparison by gender, P-valuec |
| Stimulants | 4 (3%) | 45 (41%) | P < 0.001 | 0 | 6 (33%) | P = 0.03d | P = 0.61 |
| SSRI | 7 (6%) | 43 (40%) | P < 0.001 | 0 | 8 (44%) | P = 0.008d | P = 0.80 |
| Anti-psychotic | 8 (7%) | 10 (9%) | P = 0.002d | 0 | 1 (5%) | P = 1.00d | P = 1.00 |
| Alpha agonist | 5 (4%) | 10 (9%) | P = 0.002d | 0 | 0 | NC | P = 0.35 |
- NC, not calculated (no diagnoses in particular group).
- a Number is variable depending on number prescribed prior to clinic visit; all children prescribed prior to clinic visit continued to be classified as such.
- b Based on McNemar chi-squared test for paired data.
- c Fisher's exact test.
- d Number of discordant pairs is less than 20, so exact results were used.
DISCUSSION
Given the Georgia population demographic in 2014 in which approximately 59.7% of births are White, 30.5% Black, 8.8% Hispanic, and 3.2% Asian (http://www2.census.gov/geo/maps/dc10_thematic/2010_Profile/2010_Profile_Map_Georgia.pdf), our clinic demographic reflects a considerably lower than expected frequency of patients from non-Caucasian ethnicities, except for Asians. Studies have shown that the diagnosis of FXS may take a long time to recognize and diagnose regardless of race; thus, it will be important for future studies to understand the potential contributing factors for these differences [Bailey et al., 2009; Visootsak et al., 2012].
Although there was some variability for males and small numbers for females, there was an average of a little over 2 years between first parental concerns and a FXS diagnosis. Diagnostic delay might be expected to be longer in females who are usually more mildly affected, however as these females were diagnosed because they were symptomatic early in life, they likely represent a group with symptoms on the more severe end of the spectrum for females. The diagnostic delay in this study was similar to findings (24.3 months) from the Our Fragile X World Survey [Bailey et al., 2009]. Most families in that study, as well as families in a focus group setting [Visootsak et al., 2012], reported that it took at least several visits to a health care professional before Fragile X testing is done, lengthening the diagnostic odyssey. This is concerning because a delay in diagnosis may delay early intervention services for these children, but also, importantly, delays accurate genetic counseling for the families on the recurrence risk. Approximately one third of the families in this survey went on to have a second child before a diagnosis of FXS was made, leading to the birth of a second child with FXS in many cases. Approximately 25% of families with son(s) had a second child with the full mutation before the diagnosis was given to the first child; 14 (39%) of the 36 families with daughters had a second child with the full mutation before the diagnosis. The parents reported that getting a health care provider to agree that something is wrong, and waiting for the diagnosis, were the most stressful parts of the diagnostic process [Bailey et al., 2009].
The data from the Fragile X Clinic at Emory University suggests that children with FXS are receiving appropriate assessment and treatment from their primary care providers to address their general health needs (e.g., otitis media, seizures); however, there is a gap in care to address their co-morbid behavioral issues, to implement psychopharmacologic medication management when needed, and to provide genetic counseling regarding FXS. The very large increase in diagnoses and prescriptions from our study following a Fragile X Clinic visit at Emory University suggests that these were areas of unmet need for families as the medication was only prescribed when behavioral issues were impacting the child's school, home, and social functioning and/or the child had not made progress with non-pharmacological treatments (e.g., behavioral therapy). After the visit to our Fragile X Clinic, 70% of patients were diagnosed with one of five behavioral problems, and 60% were prescribed at least one psychotropic medication. There was little difference by gender for the diagnosis of co-morbid behavioral or psychiatric problems or for psychotropic prescriptions received, again likely because the females that attended clinic were a more severely affected group. The magnitude of the burden of FXS in males is manifested in the larger absolute number of males attending the clinic (93% of the patients were male).
We also note that fragile X-specific diagnostic and treatment services was lacking prior to visiting our Fragile X clinic. The complex behavioral and comorbid problems, coupled with the need for psychotropic medications, calls for a multidisciplinary setting and approach, such as that offered by a Fragile X Clinic. Primary care doctors may not be able to undertake the management of children with FXS due to the lack of time and expertise, in particular, the monitoring of the effectiveness and the titration of psychotropic medications. Given that both the nuclear and extended family structure is affected by the condition, the Fragile X Clinic can offer a holistic approach to diagnosis, counseling, and management of FXD. Additionally, a Fragile X Clinic that provides a multidisciplinary approach to care is ideal because assessment results by each discipline are integrated to develop unified diagnostic impressions and treatment plans specific for each patient and their families’ needs. Of course, the Fragile X Clinic must work closely with the patient's primary care doctor to provide quality and comprehensive medical care to enhance quality of life for the patient and family.
This review of clinic visits also revealed the case-finding benefits of a visit to a Fragile X Clinic. From 106 initial probands with FXS, there was a threefold discovery of family members with either a premutation or full mutation. This result is similar to a previous study which revealed that after the initial diagnosis of a proband with FXS, on average at least five additional family members were diagnosed with an FMR1 mutation [Visootsak et al., 2014]. The standard practice of taking a genetic pedigree resulted in cascade screening, an active process to find immediate and extended family members affected with inherited genetic conditions, for which interventions may exist to aid individuals after the case-finding. While cascade testing for some familial chronic diseases has met with general acceptance [van Maarle et al., 2001], cascade testing among families with FXS may bring up many concerns, including feelings of guilt and cascade testing in FXS, so as to find the option that best fits their situation.
Strengths and Limitations
The Fragile X Clinic at Emory University also participates in the Fragile X Online Registry With Accessible Research Database (FORWARD), a longitudinal study of the natural history of FXS, with enrollment from at least 25 FXCRC Clinics as of this publication date [Sherman et al., in press]. The data presented here from the Fragile X Clinic at Emory University provides a unique perspective on children with FXS pre- and post-clinic visit and on the results of cascade testing, since FORWARD does not collect this aspect of child or family history. In addition, the Fragile X Clinic at Emory University offers an example of a prototype clinic and its experience over a time period not covered by the FORWARD data collection. However, the volume of patients in FORWARD (over 800) seen at all of the FXCRC Clinics provides substantial data with which toestimate the burden of comorbid problems and use of other therapies and services. Given the overall number of enrollees and variables collected by the FXCRC FORWARD project, analysis by gender, race/ethnicity, and age will also be richer and more precise than in this study or at any other individual FXS clinic.
Data on non-pharmacological therapies and services (e.g., speech and language, physical, and occupational therapies) was not collected before and after the clinic visit. While the need for psychotropic medications was fulfilled by the clinic, we did not have data on what other non-pharmacological treatment options were used before and after the clinic visit. Furthermore, we did not diagnose any new medical conditions at the Fragile X Clinic. This may be attributed to the primary care physician providing appropriate assessment and treatment for medical issues, or may be related to our clinic's main emphasis on behavioral issues; thus, the data is not captured in our record and abstraction. The potential support that the full-service aspects of an FXS clinic can provide might have been better shown with data on other non-pharmacologic therapies as well, which are being collected in the FORWARD project.
The experience of patients attending a clinic setting may not represent the entire experience of individuals with FXS in the community at large. Patients coming to clinic may represent the more severe end of the spectrum of symptomatology compared to individuals treated by a primary provider only (especially in the case of females). However, by examining a consecutive sample of patients attending a clinic, bias is minimized at least for the clinic cohort. We acknowledge the limitations on interpretation and analysis of our female patients with FXS due to the small sample size, but encourage other clinics to expand on our data by collaborating (i.e., as in FORWARD) to learn more about their female patients with FXS.
CONCLUSION
Specialty clinics, such as the Fragile X Clinic at Emory University and other Fragile X Clinics within the FXCRC, are important for advancing evidence-based clinical care, robust research about the natural history of the disorder, and educating the next generation of health professionals. This single-clinic data offers an example of the value of a prototypical Fragile X Clinic and the reach and benefits that can be achieved with attention to the individual and the nuclear and extended family members that are affected by FXD.
ACKNOWLEDGMENTS
We would like to thank the Emory Fragile X Clinic staff for the clinical care that has led to the collection of this important data. We would especially like to thank the families who have contributed to advancing our knowledge of FXS and FXD by attending the clinic and sharing their medical history and experiences.