Syndrome disintegration: Exome sequencing reveals that Fitzsimmons syndrome is a co-occurrence of multiple events
Abstract
In 1987 Fitzsimmons and Guilbert described identical male twins with progressive spastic paraplegia, brachydactyly with cone shaped epiphyses, short stature, dysarthria, and “low-normal” intelligence. In subsequent years, four other patients, including one set of female identical twins, a single female child, and a single male individual were described with the same features, and the eponym Fitzsimmons syndrome was adopted (OMIM #270710). We performed exome analysis of the patient described in 2009, and one of the original twins from 1987, the only patients available from the literature. No single genetic etiology exists that explains Fitzsimmons syndrome; however, multiple different genetic causes were identified. Specifically, the twins described by Fitzsimmons had heterozygous mutations in the SACS gene, the gene responsible for autosomal recessive spastic ataxia of Charlevoix Saguenay (ARSACS), as well as a heterozygous mutation in the TRPS1, the gene responsible in Trichorhinophalangeal syndrome type 1 (TRPS1 type 1) which includes brachydactyly as a feature. A TBL1XR1 mutation was identified in the patient described in 2009 as contributing to his cognitive impairment and autistic features with no genetic cause identified for his spasticity or brachydactyly. The findings show that these individuals have multiple different etiologies giving rise to a similar phenotype, and that “Fitzsimmons syndrome” is in fact not one single syndrome. Over time, we anticipate that continued careful phenotyping with concomitant genome-wide analysis will continue to identify the causes of many rare syndromes, but it will also highlight that previously delineated clinical entities are, in fact, not syndromes at all. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Careful phenotypic evaluation of clinical features with subsequent description and recognition of further patients has been a basic tenant of syndrome delineation. With the advent of improved genomic technologies, particularly exome sequencing, the identification of the underlying genetic etiology of rare syndromes is now relatively straightforward [Boycott et al., 2013]. Syndrome delineation is an important component of rare disease gene discovery, but may be a pitfall if the underlying assumptions about the syndrome are incorrect.
Fitzsimmons syndrome (OMIM # 270710), was first described by Fitzsimmons and Guilbert in 1987 in a pair of monozygotic twins as the constellation of progressive spastic paraplegia, brachydactyly with cone shaped epiphyses, short stature, dysarthria, and “low-normal” intelligence. Subsequent description of a single female child with similar features led to the eponym Fitzsimmons syndrome [Hennekam, 1994]. A second set of monozygous twins with cognitive impairment, dysarthria, progressive spastic paraplegia, and brachydactyly type E further supported the delineation of the syndrome [Lacassie et al., 1999]. In 2009, we described the sixth patient with spasticity, dysarthria, moderate cognitive impairment, borderline microcephaly, and brachydactyly with cone-shaped epiphyses [Armour et al., 2009].
We set out to identify the molecular cause of Fitzsimmons syndrome. We identified all of the available patients world-wide and using exome sequencing demonstrated that the clinical presentations in each of these families have different underlying etiologies, implying that Fitzsimmons syndrome is not a single Mendelian disorder, but multiple conditions co-incidentally segregating together.
PATIENTS AND METHODS
Patients
All authors of published patients were contacted and DNA from available patients was collected for exome analysis. Apart from the patient described in the 2009 report [Armour et al., 2009], only the set of twins from the original description by Fitzsimmons and Guilbert [1987] were available. The single female patient described by Hennekam in 1994 was lost to follow up and the twins described by Lacassie in 1999 were both deceased.
Exome Strategy and Analysis
The two families were enrolled into the FORGE (Finding of Rare Disease Genes) Canada initiative, a collaborative project with the goal of identifying novel genes for rare childhood diseases. Institutional research ethics board approval was obtained (Children's Hospital of Eastern Ontario), and the families were enrolled with informed consent. DNA was extracted by standard protocols. DNA from Family 1 and his parents [Armour et al., 2009], and one of the monozygotic twins from Family 2 [Fitzsimmons and Guilbert, 1987], was subjected to exome capture and high-throughput sequencing. Target enrichment was achieved using the Agilent SureSelect 50 Mb (V3) All Exon Kit and followed by sequencing (Illumina HiSeq 2000), generating >14 Gbp of 100 bp, paired-end reads per sample. Read alignment, variant calling and annotation was performed as outlined for previous FORGE projects [Beaulieu et al., 2014] with a pipeline based on BWA, Picard, Annovar, and custom annotation scripts. Variants were compared to those previously seen in the 1000 genomes phase 1 dataset (2012/04 release), the 6500 exomes of the NHLBI GO Exome Sequencing Project (ESP, Seattle, WA, data downloaded 2012-10-03), as well as in approximately 1000 exomes previously sequenced at the McGill University and Genome Quebec Innovation Centre. The pathogenicity of candidate missense SNVs was predicted using SIFT (Sorting Intolerant From Tolerant; http://sift.bii.a-star.edu.sg/) and PolyPhen-2 (Polymorphism Phenotyping; http://genetics.bwh.harvard.edu/pph2/).
Variant Validation
Sanger sequencing was used to validate the variants identified by WES and to evaluate segregation in the families. Validation and segregation of the c.734A>G: p.(Y245C) variant identified in the TBL1XR1 gene in the affected individual from Family 1 was performed with primers 5′- CTGTGTGCGATAAAGGAAGG-3′ and 5′-CAAAATGGCCACAACTAAGC-3′ (NM_024665). Validation and segregation of the variants identified in the SACS and TRPS1 genes in Family 2 was performed with primers 5′-TGTGAGAATAGAAAGCTGTTGTAGTAG-3′ and 5′-ATCTGCAAAACAGCACTTGG-3′ to test for the c.2338A>T:p.(K780*) variant, and 5′-TGATGAAACTGCTAATCTTTACCAC-3′ and 5′-AAATCAGCAAAGCTGGAAATG-3′ to test for the c.9346_9354dup: p.Leu3116_Pro3118dup duplication of three amino acids in the SACS gene (NM_014363); and 5′AAACTCCTGGGTTGATTTGG3′ and 5′GCCAGGGAATGGGACTTATC3′ to test for the c.2761C>T: p. (R921*) variant in the TRPS1 gene (NM_014112).
RESULTS
Clinical Features
The main clinical features and relevant details of the published patients with Fitzsimmons syndrome are summarized in Table I (reprinted with permission from AJMG). The patient from Armour et al., Family 1, was previously described in detail. Briefly, he demonstrated cognitive and motor delay since infancy. He was diagnosed at a young age with Tourette syndrome and later with an autism spectrum disorder. An unclassifiable brachydactyly was present in the hands and feet, but was most similar to Brachydactyly Type E. At his most recent assessment, age 23 years, he had mild-moderate cognitive impairment and could read/write at an elementary school level. Speech was mildly dysarthric and hypernasal. He had mild-moderate spastic paraparesis with extensor plantar response and hyperreflexia in the lower extremities as well as urinary incontinence. Sensation was preserved and he ambulated with a spastic gait. Head circumference remained unchanged at the 2nd centile, and final adult height was between the 5 and 10th centiles. MRI of the head had been performed when the patient was 10 years of age, and had revealed only a calcification in the white matter at the anterior aspect of the frontal horn of the right lateral ventricle [Armour et al., 2009]. A repeat MRI at 22 years of age showed no evolution of the calcification, nor any new intracranial findings. A chromosomal 44 K microarray performed in 2007 did not reveal any copy number changes [Armour et al., 2009]; however, a higher resolution array performed in 2014 revealed a 0.306Mb deletion, reported to be of uncertain clinical significance, at 10q21.3 at 68,173,577–68,479,262 (NCBI37/hg19). The deletion involves one gene, CTNNA3; follow-up testing on the parents revealed that it was de novo. Clinical testing for a panel of 51 genes (Hospital for Sick Kids, Molecular Genetics laboratory, Toronto, Canada) causative for hereditary spastic paraparesis was unrevealing.
| Fitzsimmons and Guilbert [1987] | Hennekam [1994] | Lacassie et al. [1999] | Armour et al. [2009] | |
|---|---|---|---|---|
| Age and gender | 20-year-old male MZ | 8-year-old female | 62-year-old female MZ | 16-year-old male |
| Neurological | ||||
| Progressive spasticity | + | + | + | + |
| Dysarthria | + | + | + | + |
| Nasal speech | + | + | + | + |
| Intellectual capacity | Low normal | Learning difficulties | Mild/moderate mental retardation | Moderate cognitive impairment |
| Skeletal | ||||
| Growth (Height) | Both at 3rd centile | <3rd centile | Twin 1: 95th centile | 5–10th centile |
| Twin 2: 25th centile | ||||
| Cone shaped epiphyses | + | + | + | + |
| Broad first digits | + | + | + | + |
| Brachydactyly involving: | • Metacarpals, particularly 4th and 5th; 2nd middle phalanx; 1st distal phalanx | • Metacarpals and phalanges particularly the 2nd middle phalanx and 1st distal phalanx | • 2nd metacarpal; 2nd proximal phalanx | • Metacarpals and phalanges particularly the distal and middle phalanges, 1st, 2nd and 5th proximal phalanges and 1st metacarpal |
| • Metatarsals, particularly 3rd, 4th, and 5th | • Short toes | • Metatarsals, particularly 4th and 5th | • Short toes | |
| Other | Pectus carinatum | Mild pectus excavatum | Pectus excavatum, incontinence, seizures in one twin | Tic disorder, strabismus |
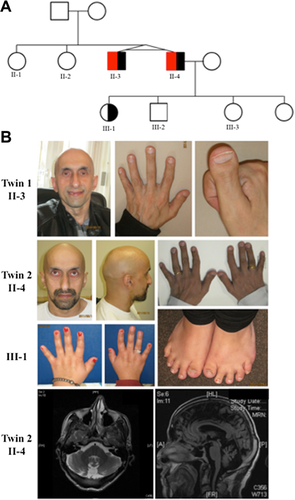
Clinical features of the twins from Fitzsimmons and Guilbert, Family 2, were previously summarized [Armour et al., 2009]. The family returned for follow-up at the initiation of this study and one of the twins had since fathered four children. Of all four children, only his eldest daughter had any similar features to her father; she manifested with a similar non-classifiable brachydactyly, but had no neurological concerns (Fig. 1, III-1). Both twins were noted to have thick eyebrows, thin/absent scalp hair, relatively large noses, a broad nasal ridge and tip with hypoplastic alae nasae, and broad columellae (Fig. 1, II-3 and II-4). Follow up MRI of one of the twins identified superior cerebellar vermis atrophy, linear hypointensities in the pons and atrophy of the cerebellar hemispheres (Fig. 1).
Exome Analysis
We first analyzed the exome sequencing data from the affected individual from Family 1 and one of the affected twins from Family 2 looking for shared variants in a single gene. No rare deleterious-appearing variants were identified in a gene shared by the two affected individuals; the exome data were then analyzed individually. In the affected individual from Family 1, one deleterious appearing potential de novo nonsynonymous variant was identified, c.734A>G, p.(Y245C) in the TBL1XR1 gene. This variant was confirmed by Sanger sequencing to be present in the patient, but not his parents (Supplemental Fig. S1). This variant was interesting as it is not present in any control databases, is a highly conserved amino acid, and de novo mutations in TBL1XR1 have been linked to autism and intellectual disability [O'Roak et al., 2012a; Saitsu et al., 2014; Tabet et al., 2014]. Therefore, for the patient from Family 1, we believe that the de novo mutation in TBL1XR1 explains his cognitive impairment and may contribute to his ASD, but we were unable to identify the cause of his spasticity and brachydactyly.
We next analyzed the exome sequencing data from one of the affected twins from Family 2. He was found to have two rare deleterious-appearing variants in the SACS gene, a known cause of autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). The first variant, c.2338A>T, p.(K780*), is predicted to cause a premature stop codon 3799 amino acids before the end of protein, while the second, c.9346_9354dup, p.Leu3116_Pro3118dup, results in a three amino acid duplication. These two SACS variants were confirmed by Sanger sequencing and segregated in seven family members. Within Family 2, only the affected twins were compound heterozygotes (Supplemental Fig. S1). Interestingly, exome analysis also identified a nonsense variant in the TRPS1 gene (c.2761C>T, p. (R921*) . The variant was validated to be present by Sanger sequencing (Supplemental Fig. S1) in both twins and in the daughter (III-3) of one of the twins. (Haploinsufficiency of this gene results in Trichorhinophalangeal syndrome type 1 (TRPS type 1) (OMIM# 190350), a rare syndrome associated with brachydactyly. Re-examination of these three individuals in light of the suspicion of two new diagnoses, ARSACS and TRPS type 1 (Fig. 1: II-3, II-4, III-1), supported the exome results and confirmed that this family has two different diseases that were conflated to represent a new clinical entity, namely Fitzsimmons syndrome.
DISCUSSION
Here, we show that despite four independent descriptions of six carefully phenotyped patients, Fitzsimmons syndrome is not caused by a single genetic etiology. The original description of the twins by Fitzsimmons and Gilbert in 1987 formed the basis for further delineation of the syndrome and we have demonstrated that these twins do not have a single genetic cause for their clinical features [Fitzsimmons and Guilbert, 1987]. Instead, they have discrete genetic etiologies for the two key features of Fitzsimmons syndrome; the spasticity and brachydactyly. Specifically, SACS mutations explain the originally documented spasticity and dysarthria, and the lack of any other family history is in keeping with the recessive nature of this condition. The spasticity associated with ARSACS is apparent early in childhood [Vermeer et al., 2003] such as occurred for these twins, whose spasticity developed by 4 years of age. The mutation detected in TRPS1 explains the brachydactyly seen in the twins and one of their offspring, which is also in keeping with the autosomal dominant nature of this condition. Follow-up review of their phenotype in light of the exome data confirms that the brachydactyly with deviation of some of the fingers at the proximal interphalangeal joints, short metacarpals with cone-shaped epiphyses, and short metatarsals observed in these patients are commonly recognized features in individuals with mutations in TRPS1 [Maas et al., 2015]. Additionally, the facial features of the twins are in keeping with those described for TRPS type 1. These genetic changes clearly explain two of the major clinical features of Fitzsimmons syndrome, the spasticity/dysarthria and the brachydactyly. No obvious cause was identified for the twins' low-normal intelligence; however, it has been postulated that cognitive deficits can present as a part of ARSACS [Verhoeven et al., 2012]. Alternatively, given the non-specificity of this finding, it may be a genetic etiology undetected by these exome studies, or secondary to other contributing genetic factors.
The single patient from Family 1 was believed to be the sixth patient from the fourth family with Fitzsimmons syndrome [Armour et al., 2009], a conclusion supported by a group of expert dysmorphologists (DW Smith Workshop 2007, Williamsburg, VA). Unlike the twins from Family 2, no clear etiology was identified for this patient's spasticity or brachydactyly. It remains entirely possible that the spasticity is non-genetic in origin or encoded by a genetic change not detectable by this technology. Certainly, this explanation is also possible for the brachydactyly as alterations of regulatory elements have been documented as causative for brachydactyly, such as described for Brachydactyly A2, which is secondary to a duplication of a downstream regulatory element [Dathe et al., 2009]. The patient's autism spectrum disorder (ASD) and cognitive difficulties may have arisen, in part or wholly, from the de novo mutation in TBL1XR1 (transducin beta-like 1 X-linked receptor-1), the protein of which aids in the recruitment of histone deacetylases to promoter regions, and has been postulated to lead to ASD and intellectual disability (ID) via haploinsufficiency [Tabet et al., 2014]. Two studies have described three individuals with de novo TBL1XR1 mutations who had ASD with varying degrees of ID [O'Roak et al., 2012a,b]. Further individuals with deletions of TBL1XR1 have been described; a fourth single individual had a de novo deletion of TBL1XR1 with mild-moderate ID and mild dysmorphic features [Tabet et al., 2014], while a mother and daughter with a TBL1XR1 deletion also shared similar mild dysmorphic features and mild-moderate ID [Pons et al., 2015], though facial features do not appear similar between the patients described in the two reports. None of these individuals were reported to have brachydactyly nor any other neurological findings. More recently however, a specific TBL1XR1 mutation, c.1337A>C (p.Tyr446Cys) was described as the cause of Pierpont syndrome, a condition with a characteristic facies, short stature, hearing loss, developmental delay as well as distinctive palmar and plantar fat pads [Heinen et al., 2016]. While the patient described herein does not share the distinctive facial features seen in Pierpont syndrome, some of the other features including short stature, short fingers and toes and developmental delay are seen in Pierpont. As such, some of the unexplained features in our patient may be explained by the TBL1XR1 mutation.
It remains unclear whether the deletion of CTNNA3 contributes to this patient's ASD phenotype; heterozygous exonic deletions of CTNNA3 do not appear to contribute to ASD, while compound heterozygous exonic deletions do [Bacchelli et al., 2014]. However, genome association studies performed on two large cohorts have suggested an association between CTNNA3 and autism [Wang et al., 2009], while whole exome sequencing in females with ASDs have implicated CTNNA3, in conjunction with other genes, as contributing to the phenotype [Butler et al., 2015]. Together, this literature suggests that at some level, this CTNNA3 CNV may be contributing to this patient's ASD.
With the findings presented herein, we propose that Fitzsimmons syndrome be dissolved as a distinct syndrome. The patients for which the syndrome was originally named, the twins from the 1987 publication, have been demonstrated to have co-occurring rare genetic conditions, while the single patient also described herein, likely has numerous, but distinct etiologies from the twins described by Fitzsimmons. The finding of multiple genetic etiologies for what was initially presumed to be a single disease has become more readily identifiable with exome sequencing [Yang et al., 2013; Yang et al., 2014; Sawyer et al., 2015]. However, we believe this represents the first example in which a syndrome that was believed to be a discrete entity, supported by multiple independent reports, and documented by OMIM as such, is proven to be the random co-occurrence of different diseases and thus not a “syndrome” at all.
At present, there remain over 1600 relatively well-described phenotypic entries in OMIM for which the molecular basis is unknown, as well as approximately 1800 entries of phenotypes for which there is suspected Mendelian basis (http://omim.org/). No doubt a subset of these entries will prove to have similar explanations, namely that they arise from co-occurrence of other known genetic diseases with hallmark clinical features that results in recognition as “distinct” when they occur together (e.g., spasticity and brachydactyly). In addition, concomitant mutations may have impacted our understanding of the clinical variability associated with recognized syndromes. For example, some reported clinical features of a recognized syndrome may in fact be a classic presentation of that disease co-occurring with an additional disorder in a particular patient; this will likely be of most significance for the rarely associated phenotypes.
Thus, while syndrome identification and delineation will always benefit from precise and careful phenotyping, it will be important to be wary of such pitfalls as we go forward.
ACKNOWLEDGMENTS
The authors would first like to thank the study patients and their families, without whose participation this work would not be possible. This work was funded by the Government of Canada through Genome Canada, the Canadian Institutes of Health Research (CIHR) and the Ontario Genomics Institute (OGI-049) (to K.M.B.). Additional funding was provided by Genome Quebec and Genome British Columbia (to K.M.B). The authors wish to acknowledge the contribution of the high throughput sequencing platform of the McGill University and Génome Québec Innovation Centre, Montréal, Canada. This work was selected for study by the FORGE Canada Steering Committee, consisting of K. Boycott (U. Ottawa), J. Friedman (U. British Columbia), J. Michaud (U. Montreal), F. Bernier (U. Calgary), M. Brudno (U. Toronto), B. Fernandez (Memorial U.), B. Knoppers (McGill U.), M. Samuels (U. de Montreal), and S. Scherer (U. Toronto). K.M.B. is supported by a Clinical Investigatorship Award from the CIHR Institute of Genetics. The authors have no conflicts of interest to declare.