Biallelic CACNA1A mutations cause early onset epileptic encephalopathy with progressive cerebral, cerebellar, and optic nerve atrophy
Abstract
The CACNA1A gene encodes the transmembrane pore-forming alpha-1A subunit of the Cav2.1 P/Q-type voltage-gated calcium channel. Several heterozygous mutations within this gene, including nonsense mutations, missense mutations, and expansion of cytosine-adenine-guanine repeats, are known to cause three allelic autosomal dominant conditions—episodic ataxia type 2, familial hemiplegic migraine type 1, and spinocerebellar ataxia type 6. An association with epilepsy and CACNA1A mutations has also been described. However, the link with epileptic encephalopathies has emerged only recently. Here we describe two patients, sister and brother, with compound heterozygous mutations in CACNA1A. Exome sequencing detected biallelic mutations in CACNA1A: A missense mutation c.4315T>A (p.Trp1439Arg) in exon 27, and a seven base pair deletion c.472_478delGCCTTCC (p.Ala158Thrfs*6) in exon 3. Both patients were normal at birth, but developed daily recurrent seizures in early infancy with concomitant extreme muscular hypotonia, hypokinesia, and global developmental delay. The brain MRI images showed progressive cerebral, cerebellar, and optic nerve atrophy. At the age of 5, both patients were blind and bedridden with a profound developmental delay. The elder sister died at that age. Their parents and two siblings were heterozygotes for one of those pathogenic mutations and expressed a milder phenotype. Both of them have intellectual disability and in addition the mother has adult onset cerebellar ataxia with a slowly progressive cerebellar atrophy. Compound heterozygous mutations in the CACNA1A gene presumably cause early onset epileptic encephalopathy, and progressive cerebral, cerebellar and optic nerve atrophy with reduced lifespan. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
The CACNA1A gene encodes the pore-forming alpha-1A sub-unit of the calcium channel Cav2.1 acting as an ion pore and a voltage sensor [Vahedi et al., 2000; Strupp et al., 2007]. Several heterozygous mutations within this gene, including nonsense mutations, missense mutations and expansion of cytosine-adenine-guanine repeats, lead to variable clinical phenotypes—including episodic ataxia type 2 (EA2 OMIM #108500), familial hemiplegic migraine type 1 (FHM1, OMIM #141500), and spinocerebellar ataxia type 6 (SCA6, OMIM #183086) [Strupp et al., 2007]. Most patients ultimately develop progressive ataxia and cerebellar atrophy [Baloh et al., 1997; Damaj et al., 2015]. A CACNA1A-related epileptic encephalopathy with generalized epilepsy and a cognitive impairment and autism has also been reported [Damaj et al., 2015].
Here we describe two children with early onset epileptic encephalopathy, progressive cerebral, cerebellar, and optic nerve atrophy caused by novel biallelic mutations in the CACNA1A gene with semidominant inheritance.
CLINICAL REPORTS
Index Patient
He was born from uneventful labor as the sixth child of non-consanguineous Estonian parents (Fig. 1a, II:7). The pregnancy was complicated by a polyhydramnios.
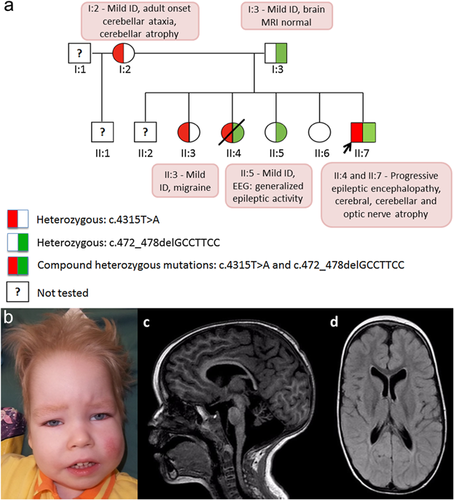
At 4 months of age, he was hospitalized due to tremulous movements in his arms and legs. His OFC was 44 cm (+3 SD), weight 6.4 kg (+1 SD), and length 62 cm (+0.5 SD). He had mild dysmorphic features (Fig. 1b). There was no eye contact; eye movements were dysconjugate with occasional episodes of nystagmus. He was observed having intensive salivation. He was markedly hypotonic with absent deep tendon reflexes and a negative Babinski sign; no head control and almost no spontaneous movements. The electroencephalogram (EEG) was abnormal with a high-voltaged up to 300 microV asymmetrical slow 2–3 Hz background activity accompanied by the multifocal sharp waves and spike-wave complexes; there was no difference between awake and sleep state. The brain MRI showed a small corpus callosum, dilatation of the frontal horns of the lateral ventricles, and diffuse hypomyelination. The magnetic resonance spectroscopy (MRS) was normal (lactate, N-acetylaspartic acid, choline, creatine, and myo-inositol). Optic atrophy was noticed in this age.
At 3 years of age his EEG showed frontal triphasic waves and the brain MRS indicated a decreased N-acetylaspartic acid to creatine ratio (1.53) and slightly increased myo-inositol (1.1). The last MRI at the age of 4 years showed an atrophic cerebellum and moderate cerebral atrophy (Fig. 1c). In the occipital and parietal regions, a hyperintense white matter signal on T2 images with concomitant decreased volume was present (Fig. 1d).
At the age of 5 years, he was blind and bedridden, had developmental delay, marked muscular atrophy and rigidity, and Friedreich-like foot deformity on his left foot. He had some overt seizures (tonic spasms) with high voltage continuous irregular epileptiform discharges and did not display an age-appropriate sleep pattern on EEG. His development was arrested; he recognized voices of his family members and turned himself onto his side. He had to be fed, but was able to swallow.
Siblings
The third child in this family, a girl (II:4, Fig. 1a) died at the age of 5 years due to epileptic encephalopathy. She was first hospitalized at the age of 4 month due to seizures. Her development corresponded to the age of 1 month with no emotional contact and extreme muscular hypotonia. Her phenotype and clinical course including MRI findings and optic nerve atrophy were very similar to the index patient. Her EEG was abnormal with multifocal interictal epileptiform discharges and ictal electric discharges.
The oldest daughter (II:3) in the family had mild intellectual disability (ID) (full scale IQ 59) diagnosed by WISC-III testing (details in Supplementary file and Supplementary Fig. S3). Occasionally, she complained of a migraine and since the age of 13 she had exercise-induced vertigo attacks. About a year later, she experienced the first episode of aggression and self-injury necessitating isolation to the acute unit of psychiatric department. The most recent brain MRI study was done at the age of 14 and showed no visual system abnormalities.
The third daughter (II:5) also had mild ID (full scale IQ 62 by WISC-III) (details in Supplementary file and Fig. S3) with impaired fine motor skills. She was seizure-free, but had generalized epileptiform (spike and slow wave complexes) discharges on EEG during photostimulation and in shallow sleep. Brain MRI, done at the age of 11 years, was normal.
The youngest daughter (II:6) had normal intellectual development. There was no clinical information of the mother's two elder sons (II:1 and II:2).
Parents
The index patient's family history was complicated due to mild ID and poor social adjustment of both parents (performance IQ 73 in the father and 63 in the mother by WAIS-III) (details are in Supplementary file and Fig. S3). Two years before the index patient was born, the mother (I:2) was diagnosed with probable alcohol-induced cerebellar ataxia—intention tremor of the hands, poor balance, and wide-based gate with horizontal nystagmus. Her brain MRI images showed cerebellar atrophy predominately affecting the vermis. The father (I:3) had no ataxia, migraines, or seizures. He had only modest complaints of rare headaches (without hemiplegia) and impairment of short-term and working memory. Brain MRI images were normal.
METHODS AND RESULTS
This study was approved by Research Ethics Committee of the University of Tartu (approval date 17/11/2014 and number 242/M-10). Exome sequencing (ES) identified two previously unreported heterozygous mutations in the index patient's CACNA1A gene: c.4315T>A p. (Trp1439Arg) in exon 27, and c.472_478delGCCTTCC p. (Ala158Thrfs*6) in exon 3 (RefSeq NM_023035.2). Confirmation of the CACNA1A mutations was performed by Sanger sequencing (Supplementary Fig. S1). The details of the ES analysis and other investigations (metabolic tests, chromosomal microanalysis, and muscle biopsy) are described in Supplementary File and Supplementary Figure S2.
The missense mutation c.4315T>A is predicted pathogenic by all tested software tools such as PolyPhen-2 [Adzhubei et al., 2010] (HumDiv score 1), SIFT [Kumar et al., 2009] (score 0), and CADD [Kircher et al., 2014] (phred score 27.1). The amino acid position Trp1439 is conserved across species with tryptophan residue being present to C. elegans (PhyloP score 4.48), and is located in ion transport domain (InterPro IPR005821). The frameshift deletion is predicted to cause a truncation of the protein in the exon three and therefore is apparently a loss-of-function mutation. Neither variant was present in ExAC database of 60,706 individuals, our in-house next generation sequencing database of 339 individuals, HGMD professional, or ClinVar. The missense mutation can be classified as likely pathogenic (class 4) and the frameshift mutation as pathogenic (class 5) according to guidelines by American College of Medical Genetics and Genomics (Supplementary file) [Richards et al., 2015].
Both mutations were discovered also in the deceased sister (II:4). Their mother (I:2) and the oldest sister (II:3) were heterozygous for c.4315T>A, and the father (I:3) together with the second living sister (II:5) were heterozygous for c.472_478delGCCTTCC. The youngest healthy sister (II:6) has neither of the mutations.
DISCUSSION
Here we present for the first time two sibs with compound heterozygous mutations in the CACNA1A gene. We conclude that these semidominantly inherited mutations in the CACNA1A gene are likely to cause early epileptic encephalopathy, progressive cerebral, cerebellar, and optic nerve atrophy with severe muscular hypotonia and reduced lifespan. Our hypothesis is supported by the family segregation of these mutations (Fig. 1a).
There are 145 disease mutations reported in the CACNA1A gene by the professional edition of Human Gene Mutation Database (HGMD®) [Stenson et al., 2009]. All these variants were in the heterozygous state and no homozygous or compound heterozygous pathogenic mutations have been reported.
Our conclusion is also supported by the experimental studies on knockout (KO) mice. Mice that completely lack neuronal Cav2.1 channel are born alive but have a severe phenotype of chronic dystonia and die soon after birth [Hoffman, 2001]. Studies with KO mice have showed that loss of Cav2.1 channel upregulates other voltage-gated calcium channels like Cav2.2 and Cav1.2 at most central synapses, although with different efficiency. This leads to synaptic dysfunction, which may explain very variable phenotypes caused by the same mutation. Additionally, studies with KO mice demonstrate profound neuronal loss throughout the cerebellum, causing ataxia, and dyskinesia in mice. For the review see Damaj et al. [2015]. Therefore, we can assume that this mechanism, most remarkably seen as cerebellar atrophy (Fig. 1c), has crucial role in pathogenesis of the siblings with biallelic mutations. To our knowledge, the optic nerve has not been investigated in the published mouse studies.
When comparing the clinical pictures of other family members, we can see some similarities, but also differences that also confirm the poor correlation of genotype and phenotype for CACNA1A mutations. The mother (I:2) is a heterozygote for a novel missense mutation c.4315T>A. Her ataxia started in adulthood and can be attributed to EA2. Her oldest daughter (II:3) is heterozygous for the same mutation, but she had the first signs of clumsiness and ataxic movements at the age of 13 with no visual changes in brain MRI that are often described in EA2 [Mantuano et al., 2010]. At the age of 16, the main problems for her (II:3) were uncontrollable episodes of aggression and self-injury requiring psychiatric admissions. Psychiatric disorders with social phobia and anxiety are frequent findings in EA2 cohorts, these symptoms have been noticed especially when the disease started early in life [Nachbauer et al., 2014]. The mother (I:2) has never had psychiatric problems other than chronic alcohol abuse.
The father (I:3) is heterozygote for c.472_478delGCCTTCC, presumably causing a translational frameshift. He shares the same mutation with one of his living daughters (II:5), they both had mild ID and no pathologic findings on brain MRI. Surprisingly, this daughter (II:5) has generalized epileptic activity seen in an EEG at the age of 11, but no overt seizures. Kinder et al. have described the same finding in a patient with EA2 with normal intelligence [Kinder et al., 2015]. Unfortunately, we do not have information about the father's EEG.
In conclusion, this report provides insight into the mutations in the CACNA1A gene and resulting phenotypes and presents a novel inheritance pattern for this disorder.
ACKNOWLEDGMENTS
We thank the family for their cooperation. This work was supported by the Estonian Research Council grant PUT355. We thank Pascal Joset from Institute of Medical Genetics, University of Zurich for his help.
WEB RESOURCES
ClinVar, www.ncbi.nlm.nih.gov/clinvar/
ExAC Browser (Beta), Exome Aggregation Consortium, http://exac.broadinstitute.org/
HGMD® Human Gene Mutational Database professional, http://www.biobase-international.com/
InterPro: protein sequence analysis & classification http://www.ebi.ac.uk/interpro/
OMIM, Online Mendelian Inheritance in Man, http://omim.org/