Maternal somatic mosaicism of FOXF1 mutation causes recurrent alveolar capillary dysplasia with misalignment of pulmonary veins in siblings
TO THE EDITOR
Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMVP; MIM#265380) is a rare but lethal developmental lung disease characterized by immature acinar structure and malformed pulmonary vasculature. Currently, definitive diagnosis is based on histological examination of the lung tissue [Cater et al., 1989; Cullinane et al., 1992]. About 80% of patients have associated extrapulmonary anomalies including cardiovascular, genitourinary, and gastrointestinal malformations [Sen et al., 2004]. In 2009, heterozygous deletions and point mutations of the FOXF1 gene were identified as the cause of ACDMVP [Stankiewicz et al., 2009]. FOXF1 gene is located at chromosome 16q24.1 region and the protein product, FOXF1, is highly expressed in fetal and adult lung tissues [Hellqvist et al., 1996]. However, FOXF1 genomic aberrations were identified in only 70% of ACDMVP cases [Sen et al., 2013a], the underlying cause of remaining cases was unknown. Up till now, there were 100 ACDMVP cases reported in the literature. The majority were sporadic with only 10% being familial with affected siblings. All reported familial cases were maternally inherited. This suggested that FOXF1 gene is paternally imprinted in human lung tissues [Sen et al., 2013b; Szafranski et al., 2013]. And FOXF1-related ACDMVP is an evolving novel human imprinting disease.
We here reported a familial case of ACDMVP with affected siblings caused by a reported FOXF1 gene mutation [Sen et al., 2013a] that was maternally inherited. The mother was somatic mosaic mutation carrier confirmed by molecular testing, which has never been reported in the literature.
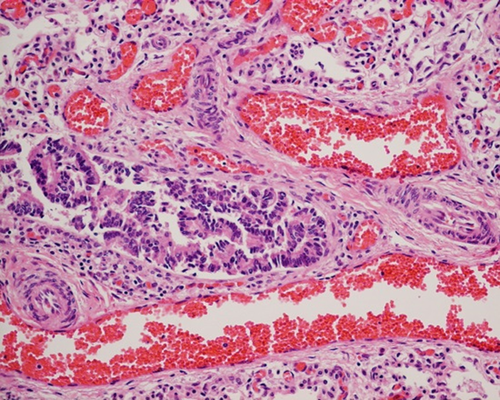
The male newborn was the second child of an Asian/Caucasian couple. He was born at 37 weeks 5 days gestation with birth weight of 3.7 kg by Caesarean section. The Apgar score was 7 at 1st minute and 8 at 5th minute. Antenatally, he had suspected absence of gallbladder and polyhydramnios since 28 weeks gestation. Soon after delivery, the baby developed respiratory distress that required intubation and mechanical ventilation at 2 hr of life. Chest X-ray showed bilateral ground glass appearance with a small rim of pneumothorax. Echocardiogram showed secundum atrial septal defect, dilated right atrium and ventricle, and right-to-left shunt through patent ductus arteriosus, suggestive of suprasystemic pulmonary blood pressure. There was significant pre- and postductal difference in oxygen saturation. The clinical picture was compatible with persistent pulmonary hypertension of newborn (PPHN). He was then put on high frequency ventilation, inhaled nitric oxide and multiple inotropic agents. However, the hypoxemia and hypercarpnia continued to deteriorate such that he eventually succumbed on day 2 of life. Postmortem examination was performed, which showed normal gallbladder and without major congenital malformation. However, the lung biopsy showed malposition of large ectatic pulmonary vein with increased muscularization of pulmonary arteries (Fig. 1). This confirmed the diagnosis of ACDMPV.
The family history was significant that the previous child of this couple also had ACDMPV confirmed histologically. He was born at 38 week gestation with birth weight of 3.6 kg. Antenatally, he had omphalocele diagnosed at 13 week gestation. Despite intensive ventilator support, he died at 33 hr of life due to refractory hypoxemia. Postmortem examination showed he had omphalocele, gut malformation, and ACDMPV on lung biopsy.
As there was recurrence of ACDMPV among this couple, the family was then referred to our clinical genetic clinic for assessment. Based on the clinical and histological diagnosis, FOXF1 gene related disease was suspected. However, DNA was not available in the first child that genetic testing could only be performed for the second child. A heterozygous missense variant c.294C>A (p.His98Gln) in exon one of the FOXF1 gene (Reference sequence: NM_001451.2) was detected. This variant was located at the DNA binding domain of the FOXF1 gene, which was highly evolutionarily conserved among different species. Furthermore, it has not been reported as a normal variant in Single Nucleotide Polymorphism Database (dbSNP), 1,000 genome project, Exome Aggregation Consortium (ExAC), NHLBI GO Exome Sequencing Project (ESP). In silico amino acid substitution using Alamut programme by algorithm SIFT and Mutation taster suggested as deleterious nature. Overall, this mutation was classified as likely pathogenic according to the latest 2015 ACMG guideline.
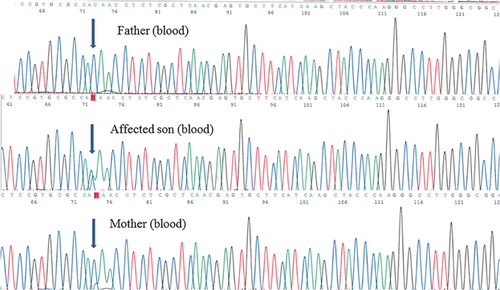
Parental study showed that the mother, who was clinically normal without pulmonary, cardiac, gastrointestinal, or genitourinary disease, also carried this variant in her peripheral blood sample. However, the DNA electropherogram suggested somatic mosaicism because of the weaker signal intensity of the mutant (Fig. 2). The somatic mosaicism in mother was subsequently confirmed by quantitative PCR method. Using different tissues, the mutant load was found to be 28.7%, 37.3%, and 40.3% in her peripheral blood, hair follicle, and buccal swab specimens, respectively. In contrast, the mutant load in the peripheral blood of the affected second child was 52%, compatible with constitutional heterozygous mutation.
FOXF1 is a transcription factor that is highly expressed in lung mesenchymal tissue during fetal development [Mahlapuu et al., 1998]. It plays an important role in cell migration during embryogenesis [Malin et al., 2007]. Therefore, it is essential for pulmonary alveolar and vasculature development. Heterozygous point mutations and genomic deletions at chromosome 16q24.1 region encompassing the FOXF1 gene have been reported to be associated with ACDMPV [Stankiewicz et al., 2009]. The initial proposed mechanism was loss of function or haploinsufficiency of this dosage sensitive FOXF1 gene. However, the molecular pathogenesis was far more complicated as observed in familial cases.
Although most ACDMPV cases were sporadic, about 10% were familial. In these familial cases, all FOXF1 genomic aberrations were maternally inherited, suggesting that the gene was paternally imprinted. By genomic mapping, a 60 kb evolutionarily conserved and differentially methylated cis-regulatory enhancer region was identified 272 kb upstream of FOXF1 gene [Stankiewicz et al., 2009] that was physically interact with FOXF1 promoter. Maternal deletion of this upstream region resulted in ACDMVP [Szafranski et al., 2014]. However, the imprinting phenomenon was incomplete. As there was usually normal pulmonary phenotype in patient with paternal uniparental disomy 16, therefore, FOXF1 expression from two paternal chromosome 16 was proposed adequate to maintain its functional requirement [Sen et al., 2013b], and the exact pathogenesis of FOXF1 imprinting effect was still unknown.
This family had illustrated the parent-of-origin effect of FOXF1 mutations in ACDMVP. The recurrence risk of ACDMVP among siblings in this couple could be increased up to 50% that depends on the level of maternal gonadal mosaicism. This is important for genetic counseling and would affect the future reproductive options for this couple.
In conclusion, we have reported the first familial case of ACDMVP as a result of maternal somatic mosaic FOXF1 mutation, which provided additional evidence for this novel imprinting disorder. However, further studies are necessary to decipher the imprinting phenomenon on chromosome 16q24.1 and the epigenetic mechanisms that regulate FOXF1 expression.