Three cases of Troyer syndrome in two families of Filipino descent
Abstract
Troyer syndrome is a complex hereditary spastic paraplegia (HSP) due to a mutation in SPG20 first reported in the Old Amish population. A genetic mutation in SPG20 is responsible for a loss of function of the protein spartin in this disease. Since its initial report, this syndrome has also been reported in Turkish and Omani families. Here we report the case of three patients of Filipino descent with Troyer syndrome. Whole exome sequencing (WES) identified a homozygous mutation c.364_365delAT which predicts p.Met122Valfs*2 in SPG20. This is the same mutation identified in affected patients from the Omani and Turkish families, and is the first report of this syndrome in the Filipino population. Although Troyer syndrome has characteristic phenotypic manifestations it is likely underdiagnosed due to its rarity and we expect that WES will lead to identifying this disease in other individuals. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Hereditary spastic paraplegias (HSP) are a group of genetic neurodegenerative disorders which are characterized by progressive weakness and spasticity of the lower limbs [Lo Giudice et al., 2014]. These disorders can be further categorized into pure and complex subtypes. Complex HSP, such as Troyer syndrome (OMIM#275900), are typically associated with additional neurological features such as extrapyramidal symptoms, intellectual disability, and skeletal abnormalities [Proukakis et al., 2004]. Pure HSP do not exhibit these additional features. Most complex HSP are inherited in an autosomal recessive pattern, however they may also be inherited in an autosomal dominant, X-linked recessive, or mitochondrial manner [Hensiek et al., 2015]. Additionally, sporadic de novo mutations are common [Lo Giudice et al., 2014].
Troyer syndrome was first reported in the Old Order Amish in 1967 [Cross and McKusick, 1967]. Clinical features of this disorder include progressive spastic paraparesis, dysarthria, pseudobulbar palsy, distal amyotrophy, developmental delays, and short stature [Patel et al., 2011]. The causative mutation, a single nucleotide deletion c.1110delA which predicts p.K370Nfs*30 in SPG20 (NM_015087), was first identified in 2002 [Patel et al., 2002]. SPG20 encodes the protein spartin, and this mutation has been shown to result in a complete loss of protein function [Bakowska et al., 2008]. Since this initial report, this disorder has been reported in two other populations. In 2010, Manzini et al. [2010] reported two related Omani families with HSP and features consistent with Troyer syndrome found to have a pathogenic c.364_365delAT which predicts p.Met122Valfs*2 in SPG20 [Manzini et al., 2010]. In 2015, Tawamie et al. reported two additional cases in a family of Turkish origin due to the same p.Met122Valfs*2 mutation in SPG20 [Tawamie et al., 2015]. In 2015, Alazami et al. reported a different frameshift mutation c.1450_1451insA which predicts p.T484Nfs*13 in SPG20 within a consanguineous Saudi family [Alazami et al., 2015]. However, these patients were reported to have speech and motor delay, tremors, microcephaly, and strabismus which are not consistent with the phenotype previously reported with Troyer syndrome. Here we report the case of two sisters as well as an unrelated patient, all of Filipino descent, with clinical features of Troyer syndrome found to be positive for the p.Met122Valfs*2 mutation in SPG20. The content of this manuscript is not considered research at our institution and instead falls in the realm of routine clinical care.
CLINICAL REPORT
The first two patients presented here are siblings, an 8-year-old female patient 1 (P1, Fig. 1A) and a 6-year-old female patient 2 (P2, Fig. 1B) born to Filipino parents. They were referred to genetics after evaluation for failure to thrive, short stature, and speech delay. Patient 1 was born by spontaneous vaginal delivery at 35 weeks gestation, with BW of 1.96 kg (10th centile). There were no known exposures to illnesses, infections, tobacco, alcohol, or drugs during pregnancy. She spent 1 day in the NICU for supplemental oxygen related to respiratory distress at birth, but was discharged home with her mother. Her developmental delay was first evident when she sat late. She walked at age 2 years. Patient 1 was evaluated by endocrinology for short stature and was receiving growth hormone therapy with some response noted. Patient 1's physical exam was significant for height and weight less than the 3rd centile, microcephaly (OFC 2nd centile), frontal bossing, and dystonic posturing of fingers (Figs. 1A and 2A). She had oromotor dysfunction and drooling. Speech was significant for both palatal and lingual dysarthria. Patient 1 did not display any spasticity in her arms. Both ankle cords were tight, and brisk deep tendon reflexes were present, more pronounced in the legs than the arms, with a few beats of clonus at both ankles. Gait and stance were abnormal, with inturning of the left more than right ankle and inversion with gait (Fig. 2A). Patient 1 had mild proximal muscle weakness with strength slightly decreased in the biceps, deltoids, and hip flexors. She experienced tremors in both upper extremities when attempting to hold objects. All other physical and neurological features were otherwise normal. She was in the 3rd grade in a special needs class with an Individual Education Plan (IEP) in place. She was reading at grade level but having some difficulty with math. She was receiving speech, occupational and physical therapy once weekly. Patient 1 was evaluated by neurology for hypotonia and had a metabolic work-up with normal urine organic acids and lactate. Plasma amino acids showed elevations in serine, leucine, isoleucine, and lysine which were non-specific. Ammonia was mildly elevated at 74 umol/L (normal 10–52 μmol/L). Brain magnetic resonance imaging (MRI) revealed increased T2/FLAIR signal within the ventrolateral thalami and posterior limb of the internal capsule, which was stable with serial imaging. No elevated lactate spike was identified on MR spectroscopy. Spine MRI was normal. Osseous survey was not performed.
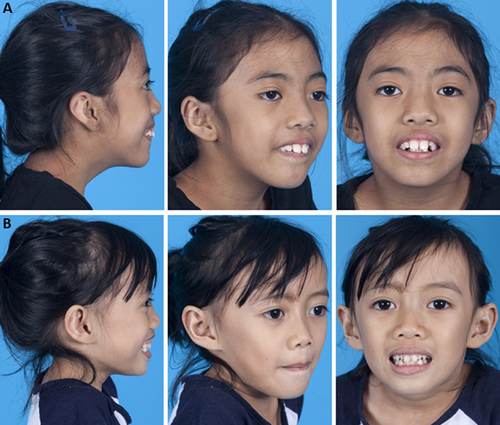

Patient 2 was born by spontaneous vaginal delivery at term with BW 2.07 kg (<3rd centile). There were no known exposures to illnesses, infections, tobacco, alcohol, or drugs during pregnancy. She was discharged home with her mother soon after delivery. She was similarly affected with failure to thrive, short stature, and speech delay. Patient 2's physical exam was significant for height and weight less than the 3rd centile, microcephaly (OFC 2nd centile), and frontal bossing (Figs. 1B and 2B). She had dystonic posturing of her left fingers and left wrist (Fig. 2B). Patient 2 also had oromotor dysfunction with significant palatal and lingual dysarthria. She did not display any spasticity in her arms but both ankle cords were mildly tight, with brisk deep tendon reflexes, more pronounced in the legs than the arms. Gait and stance were abnormal, with inturning of the left more than right ankle and inversion with gait (Fig. 2B). She also had mild proximal muscle weakness with strength slightly decreased in the biceps, deltoids, and hip flexors. Family reported she experienced fatigue after walking long distances. All other physical features were otherwise normal. She was performing at grade level, and was not experiencing any difficulties with learning. An osseous survey was normal except for mild midface hypoplasia. Karyotype showed 46,XX. Single nucleotide polymorphism (SNP) chromosomal microarray was normal. Brain MRI was not performed.
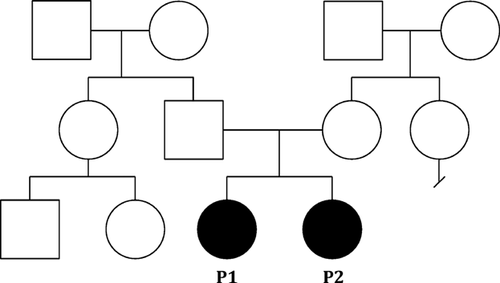
The patients' mother is 59 inches tall and the patients' father is 69 inches tall. The maternal side of the family is of generally short stature. There is no known history of birth defects, intellectual disability, genetic conditions, recurrent miscarriages, or sudden unexplained deaths in either side of the family (see pedigree Fig. 3). Both sides of the family are from the Philippines and consanguinity was denied.
Patient 3 (P3) is an unrelated 8-year-old male. He was born by cesarean delivery due to breech presentation at 37 weeks gestation with BW 2.7 kg (20th centile). There were no known exposures to illnesses, infections, tobacco, alcohol, or drugs during pregnancy. He was discharged home with his mother soon after delivery. Patient 3 had been followed by endocrinology from the age of 5.5 years for short stature and was receiving growth hormone therapy with good response. His parents reported decreased strength, balance problems, and slurred speech when he was first evaluated in genetics clinic at age 7 years. These abnormalities were first noticed at the age of 3 years. Patient 3's physical exam was significant for height and weight less than the 3rd centile, microcephaly (OFC 2nd centile), a prominent forehead with a flat midface and narrow mandible, and a single transverse palmar crease on the right. He exhibited drooling and dysarthria, mild proximal muscle weakness with strength decreased in the trunk/abdominals and arms and a wide based wobbly gait. He did not display any spasticity. All other physical features were otherwise normal. He was in the 2nd grade in a special needs class with an IEP receiving speech, occupational and physical therapy. Parents reported normal cognition. Brain MRI was normal. Osseous survey was not performed.
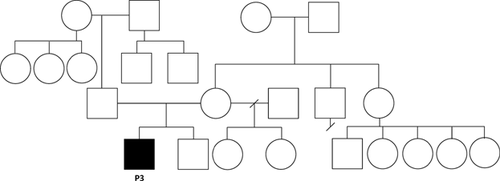
The patient's mother is 64 inches tall and the patient's father is 71 inches tall. There is a maternal grandmother with hearing impairment and a maternal half-sister with a history of febrile seizures. There is no known history of neurological disorders, short stature, intellectual disability, behavioral disorders, infant death, or recurrent miscarriages in either side of the family (see pedigree Fig. 4). The patient's mother is Filipino and father is of Filipino, Chinese, Italian, and Spanish descent. Consanguinity was denied. Table I shows the features of P1, P2, and P3 in comparison with prior reported cases.
| Characteristics | Old Amish, n = 21 | Omani, n = 6 | Turkish, n = 2 | Filipino, n = 3 |
|---|---|---|---|---|
| Mean age at examination (y) | 30 | 17 | 22 | 7 |
| Microcephaly | − | − | + | + |
| Short stature | + | + | + | + |
| Spastic paraparesis | + | + | + | ±a |
| Distal amyotrophy | + | + | + | + |
| Dysarthria | + | + | + | + |
| Emotional lability | + | − | + | − |
| Motor delay | + | + | + | + |
| Speech delay | + | + | + | + |
| Learning difficulty | + | + | + | + |
| Skeletal abnormalities | + | + | + | − |
| Gait abnormalities | + | + | + | + |
| Brain MRI abnormalities | + | + | − | ±a |
| SPG20 mutation | c.1110delA | c.364_365delAT | c.364_365delAT | c.364_365delAT |
- +, present; −, absent.
- a No brain MRI abnormalities or spastic paraparesis in P3.
MOLECULAR STUDIES
Untargeted, whole exome sequencing (WES) with full analysis of all characterized disease genes was performed on the trio of P2, her mother and her father. Mean coverage was 90.78%. Patient 2 was found to be homozygous for an alteration c.364_365delAT which predicts p.Met122Valfs*2 in SPG20. Biparental inheritance was confirmed by Sanger sequencing with each parent being heterozygous for this mutation. This frameshift mutation was classified as pathogenic. Co-segregation analysis by Sanger sequencing revealed P2's similarly affected sister (P1) was also homozygous for this mutation. A FOXH1 variant was also found to be homozygously present but clinically filtered out as reported disease in this gene is associated with human heart defects and holoprosencephaly [Roessler et al., 2008]. TTN variants were also found to be present in a compound heterozygous manner but in silico analyses of the variants were also inconsistent in their prediction. Since recessive TTN truncating mutations have been associated with centronuclear myopathy [Ceyhan-Birsoy et al., 2013], we cannot fully exclude the possibility that these variants found in TTN have any phenotypic contribution to our patients at this time. TTN mutations have also been linked to cardiomyopathy which are not present in our patients or their families. There were no de-novo or x-linked variants identified. Whole exome sequencing was separately performed on the trio of P3, his mother and his father. Mean coverage was 89.98%. Patient 3 was also found to be homozygous for the same p.Met122Valfs*2 mutation in SPG20 with each parent heterozygous. There were no other homozygous variants found. IGF2R and MYO1F variants were found to be present in a compound heterozygous manner. There were no de novo or x-linked variants identified. IGF2R mutations [De Souza et al., 1995] have been linked to somatic hepatocellular carcinoma and MYO1F variants [Chen et al., 2001] have shown a possible association with hearing loss which do not correlate clinically with the presentation of our patients. Both patients' samples were prepared with SeqCap EZ VCRome 2.0 (Roche NimbleGen) capture and sequenced using paired-end, 100 cycle chemistry on the Illumina HiSeq 2500.
DISCUSSION
The patients presented here carry the same mutation that has been identified in an Omani family and two siblings from a Turkish family [Manzini et al., 2010; Tawamie et al., 2015]. Just as the patients described from the Omani and Turkish families, our patients presented with short stature, developmental delays, distal amyotrophy, and spasticity. A comparison of the features seen in these patients can be seen in Table I. There are a few differences between these patients with the p.Met122Valfs*2 mutation in SPG20 and the Amish cohort which may be related to genetic or epigenetic influences. Specifically, patients in the Amish cohort were not noted to have any dysmorphic facial features. They were however noted to have emotional lability, whereas this feature was only found in one of the Turkish patients with the p.Met122Valfs*2 mutation. Only three brain MRIs were available for patients from the Amish cohort, and they were found to have abnormal T2 hyperintense signal in the periventricular white matter and the posterior limb of the internal capsule [Proukakis et al., 2004]. Atrophy of the cerebellar vermis and periventricular white matter hyperintensity on T2-weighted images was reported in the Omani population [Manzini et al., 2010]. Patients from the Turkish cohort had no apparent brain MRI abnormalities [Tawamie et al., 2015]. Patient 1 was found to have mild brain MRI abnormalities and brain MRI was not performed on P2. Patient 3 had a normal brain MRI.
Based on the phenotypic similarities between our patients and the previously described subjects from the index Omani and Turkish families, we infer this mutation in SPG20 is the disease causing mutation. The SPG20 gene is located on chromosome 13q12.3 and encodes the spartin protein [Patel et al., 2002]. This protein has been shown to be absent in multiple cell lines of patients affected by Troyer syndrome, suggesting a loss of function disease mechanism [Bakowska et al., 2008]. It is possible that the phenotypic differences seen between the Amish cohort, the Omani and Turkish cohort and those reported by Alazami et al. [2015] are due to differences in the position of the causative mutations and their effects on protein stability or function. Patients carrying the p.K370Nfs*30 or p.Met122Valfs*2 mutation have no protein expression detected, whereas those reported by Alazami et al. [2015] with a late C-terminal truncating mutation p.T484Nfs*13 may have residual protein expression which might lead to phenotypic differences. The specific function of spartin with relation to neuronal development and function is still unknown. Spartin is involved in the regulation of lipid droplet turnover, and misregulation of this process can affect the function of motor neurons [Eastman et al., 2009]. It has been shown to contribute to the formation of dendritic aggresome-like induced structures (DALIS) through a ubiquitin-binding region [Karlsson et al., 2014] and cells depleted of spartin have shown impaired cytokinesis [Yang et al., 2008; Renvoise et al., 2010]. A recent animal model of a null mutation in SPG20 has also demonstrated a sensitivity to oxidative stress [Truong et al., 2015].
In this case, WES revealed a diagnosis which otherwise may not have been recognized given the rarity of this condition which had previously been reported only in other racial/ethnic populations. Finding the pathogenic gene mutation is useful for guiding management, disease surveillance and treatment in patients with HSP. Our group of patients represents the third described in populations outside of the original Old Amish cohort. WES will likely lead to other patients being diagnosed which will be helpful in defining the full spectrum and natural history of this condition.
ACKNOWLEDGMENTS AND DISCLOSURES
We would like to thank the families for their participation in this report. All authors have no conflicts of interest to disclose and there was no financial support. KLH and WA are full time employees of Ambry Genetics. The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, the United States Air Force, Department of Defense or the U.S. Government. We certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of the data (when applicable), the writing and revision of the document and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approval to use the information and made appropriate acknowledgements in the document; and that each takes public responsibility for it.