A splice site mutation in HERC1 leads to syndromic intellectual disability with macrocephaly and facial dysmorphism: Further delineation of the phenotypic spectrum
Abstract
We report on a sib pair of Indian origin presenting with intellectual disability, dysmorphism, and macrocephaly. Exome sequencing revealed a homozygous splice site HERC1 mutation in both probands. Functional analysis revealed use of an alternate splice site resulting in formation of a downstream stop codon and nonsense mediated decay. In the light of recent reports of HERC1 mutations in two families with a similar phenotypic presentation, this report reiterates the pathogenic nature and clinical consequences of HERC1 disruption. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
HERC1 is a giant protein believed to be involved in intracellular membrane trafficking and ubiquitinization of specific targets which play an important role in eukaryotic cell function [Garcia-Gonzalo and Rosa, 2005]. It is also presumed to play a regulatory role in the mTOR pathway through its interaction with the TSC1–TSC2 complex. Recently, two papers reported biallelic HERC1 mutations in individuals with intellectual disability and macrocephaly [Nguyen et al., 2016; Ortega-Recalde et al., 2015]. This is a report of sibs of Indian origin who were found to harbor homozygous HERC1 mutations and presented with a clinically similar phenotype.
PATIENTS AND METHODS
This research was prospectively reviewed and approved by a duly constituted ethics committee and consent was obtained to publish patient photographs.
CLINICAL REPORT
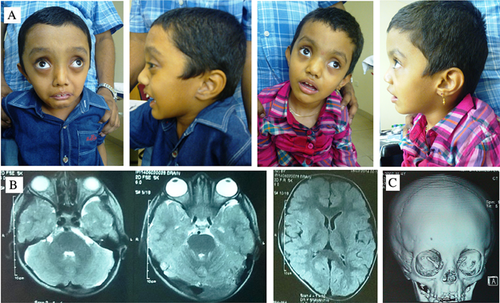
Two sibs, a 7 years old male and a 3 years old female, were born to third degree consanguineous parents originating from southern India. They were evaluated due to intellectual disability. History of the male sib revealed an uneventful perinatal period, a birth weight of 4,500 g, with height and head circumference not recorded. Subsequent developmental milestones were delayed in all spheres, with a severe degree of disability. There was no history of seizures, behavioral abnormalities or recurrent illnesses. No anthropometric records of the intervening period were available. At presentation, the head circumference was 54 cm (+2 SD), height 109 cm (−2.1 SD), and weight 16 kg (−2.5 SD). The facies showed presence of a large head and tall forehead, proptosis, upslanted palpebral fissures, notched alae nasi and low set, posteriorly angulated, large ears. Oral cavity showed prominent palatal shelves and gum hypertrophy. Generalized hypotonia was present. Mild joint laxity was appreciated with right pes planus, mild kyphoscoliosis and long, thin fingers. The external genitalia showed presence of a micropenis and bilaterally descended testes. Investigations revealed a normal peripheral blood karyotype, normal MR neuroimaging study of the brain and no internal malformations. The bone age corresponded to chronological age. CT study of skull revealed metopic suture synostosis, which was likely age related. Orbits were reported to be shallow. The younger sib was reported to have ventriculomegaly (14 mm) and macrocephaly on 36 weeks antenatal ultrasonography. Birth weight was 3 kg, head circumference and length records were unavailable. The perinatal period was uneventful. No subsequent anthropometric records were available. The child had global developmental delay since early infancy and was unable to stand or speak meaningfully. There was no history of seizures, behavioral abnormalities or recurrent illnesses. Examination revealed a head circumference of 49 cm (+0.6 SD), height of 101 cm (+2.3 SD), weight of 15 kg (+1 SD), and dysmorphic features similar to her brother. The eyes were prominent, widely spaced with upslanted palpebral fissures. The head appeared relatively large with a tall forehead. The ears were low set and alae nasi were notched. There was generalized hypotonia, mild joint laxity with a lumbar gibbus and long, slender fingers. No renal or skeletal malformations were detected on imaging. The bone age corresponded to chronological age. Parents′ head circumferences were 56 cm each. Both parents were tall, without significant health issues. The father had notched alae nasi similar to the children. No similarly affected family members were reported. Figure 1 shows the clinical and imaging findings of the sibs.
METHODS
The Phenomizer software was used for phenotype ontology and matching with known phenotypes. No hits were obtained. Chromosomal microarray was performed on the peripheral blood leukocyte DNA of the older sib using the Illumina HumanCytoSNP-12 platform (Illumina, San Diego, CA). Data analysis was performed using KaryoStudio v1.4 (Illumina) software. Size thresholds of 200 kb for deletion, 500 kb for duplication, and 1 Mb for homozygous regions were used. No significant copy number variants were detected. However, 26 homozygous regions containing 831 genes, with 85 reported to show autosomal recessive inheritance, were found. Genomic Oligoarray and SNP array evaluation tool (version 3.0) was used to look for phenotype matches with known disease causing OMIM genes, both under autosomal dominant and recessive models. No candidate gene could be ascertained.
Exome sequencing was carried out on the peripheral leucoycte DNA of both sibs to ascertain coding sequence variants. Exome capture was performed on genomic DNA sample using Agilent SureSelectXT V5 exome capture kit (Agilent, Santa Clara, CA). The libraries were sequenced to mean >80–100× coverage on Illumina HiSeq2000 platform (Illumina). The reads obtained were mapped against human reference genome (GRCh37/hg19) followed by detection of SNPs and small Indels. Variant annotation was performed using Annovar for location and predicted function. PCR was performed using specific primers F-5′-TCTGATGTGTGCTTATAGAGTTT-3′ and R-5′-AAACCAAAACACCCTGCTAG-3′ and Sanger sequencing was done using ABI 3130 Genetic analyzer (Life Technologies, Carlsbad, CA) following the manufacturer's protocol in control, mother and patient samples to validate the mutation.
Total RNA was extracted using RNeasy Mini Kit (QIAGEN, Valencia, CA) following the manufacturer's protocol. RNA integrity was checked by gel electrophoresis and concentration was assessed spectrophotometrically. Reverse transcription was carried out using SuperScript™ III First-Strand cDNA Synthesis assay system (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Primers were designed in adjoining exons (F-5′-GAGTGGAGATGTGGGGAATG-3′ and R-5′-AGGACTGGAAGCCTGAACTC-3′) around the mutation and RT-PCR was performed to amplify the region. The amplicon size was checked by agarose gel electrophoresis and analyzed by bidirectional Sanger sequencing.
Quantitative RT-PCR (RT-qPCR) was performed with the Power SYBR Green PCR Master Mix (Takara Bio Inc., Tokyo, Japan) on an Applied Biosystems 7500 Real-Time PCR System (Life Technologies) following the manufacturer's protocol. The same RT-PCR primers were used to amplify HERC1. GAPDH was used as reference gene and amplified using specific primers F-5′-GTCAAGCTCATTTCCTGGTA-3′ and R-5′-CATCTTCTAGGTATGACAACG-3′.
RESULTS
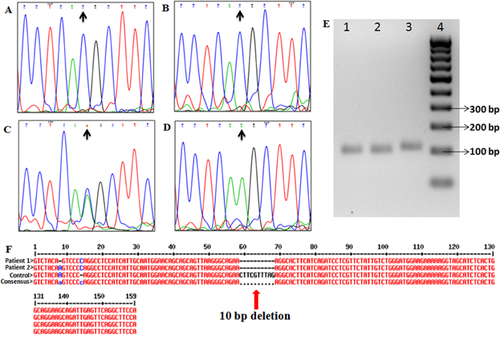
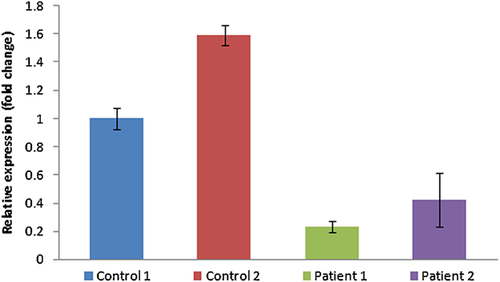
Exome of the male sib revealed a total of 25293 variants, including single nucleotide variants (SNVs) and Indels. Gross filtering using 1000 Genomes (≤0.01 MAF), Exome Variant Server (≤0.01 MAF), Exome Aggregation Consortium (≤0.01 MAF) and dbSNP databases revealed 17 homozygous unique variants including non-synonymous, splice-site and Indel variants causing frameshift. Further stringent filtering using CADD score (≥10) left only five potentially significant variants. For the female sib a total of 25,619 variants including SNVs and Indels were identified. Using the same filtering pipeline as used in the male, 13 unique homozygous variants were obtained in the female child. Stringent filtering using CADD score (≥10) identified only eight potentially significant variants. Among these variants only one variant was shared between the two siblings. This novel variant was a c.4906-2A>C 3′ splice-site mutation present in HERC1. HERC1 was present in a 12.9Mb homozygous region in the microarray data of the elder child. Sanger sequencing in both patients and their mother confirmed presence of the mutation in the children and heterozygous status in the mother. cDNA analysis in both patients revealed that this mutation results in a 10 base pair deletion in exon 27, leading to a frameshift and a premature stop codon (TGA) 75 nucleotides downstream in the new reading frame. (Fig. 2). On quantitative RT-PCR, HERC1 mRNA level was found to be at least 77% decreased in patient one and 58% decreased in patient two compared to controls (Fig. 3). This result suggests that the identified variant leads to partial nonsense mediated decay of HERC1 mRNA and that the phenotype is likely due to the decreased production as well as loss of important functional domains of the truncated protein. This variant has been submitted to the Clinvar database (Accession no. SCV000245769.1).
DISCUSSION
HERC1 is a 4861 amino acid protein with one HECT (Homologous to E6AP COOH Terminus) and two RLD (Regulator of Chromosome Condensation 1 Like Domain) domains [Garcia-Gonzalo and Rosa, 2005]. The HECT domain interacts with the TSC1–TSC2 complex and likely contributes to a regulatory role of HERC1 in the mTOR pathway by acting as an ubiquitin ligase for TSC2 and decreasing its stability [Chong-Kopera et al., 2006; Wataya-Kaneda, 2015]. The RLD domains act as GEF (guanine nucleotide exchange factor) transporters for small G proteins like ARF1 (ADP ribosylation factor 1) and Rab GTPases, bind clathrin which form part of vesicle membranes, associate with phosphatidylinositol 4–5 bisphosphate, and co-localize with actin polymers at membrane blebs. Based on this evidence, it is believed HERC1 is involved in intracellular membrane trafficking and ubiquitinization of specific targets which are fundamental to eukaryotic cell function [Garcia-Gonzalo and Rosa, 2005]. However, despite its discovery in the year 1996, and the various experiments demonstrating its interactions with other proteins, the exact physiological role of HERC1 remains unknown.
Two recent studies reported biallelic HERC1 mutations in probands of Moroccan and Columbian origin, presenting with intellectual disability and macrocephaly [Nguyen et al., 2016; Ortega-Recalde et al., 2015]. The sibs reported here are similarly affected. Their facial gestalt shares common features with the patients reported by Ortega-Recalde et al. [2015]. However, no neuroimaging abnormalities were observed in the elder sib who underwent a MRI brain. This is unlike the patient reported by Nguyen et al. [2016] who had a thick corpus callosum and cerebellar hypoplasia. One sib reported by Ortega-Recalde et al. [2015] had ventriculomegaly. A large size at birth (as reflected by the high birth weight of the elder sib), macrocephaly of antenatal onset (as reflected by the increased head size reported in third trimester antenatal ultrasound of the second sib), followed by postnatal predominant macrocephaly with normalization of weight and height and a normal bone age appears to be the pattern in these individuals. A similar clinical picture can be seen in the probands reported previously [Nguyen et al., 2016; Ortega-Recalde et al., 2015]. Table I presents a comparative overview of the clinical features in the three reports.
| Nguyen et al. [2016] | Ortega-Recalde et al. [2015], P1 | Ortega-Recalde et al. [2015], P2 | Present report P1 | Present report P2 | |
|---|---|---|---|---|---|
| Age | 18 years | 29 years | 24 years | 7 years | 3 years |
| Sex | Male | Male | Female | Male | Female |
| HC at presentation | 66.5 cm (+10 SD) | >+2 SD | >+2 SD | 54 cm (+2 SD) | 49 cm (+0.6 SD) |
| HC at birth | 37cm(+2 SD) | NR | NR | NR | Macrocephaly and ventriculomegaly on antenatal USG |
| Birth weight | 4 kg (+2 SD) | 4 kg (+2 SD) | NR | 4.5 kg (+3SD) | 3 Kg (−1 SD) |
| Birth length | 53 cm (+2 SD) | 54 cm (+2 SD) | NR | NR | NR |
| Height at presentation | 172 cm (−0.82 SD) | > + 2 SD | > + 2SD | 109 cm (−2.1 SD) | 101 cm (+2.3 SD) |
| Weight at presentation | 51 kg (−2 SD) | NR | NR | 16 kg (−2.5 SD) | 15 kg (+1 SD) |
| Intellectual disability | Severe | Severe | Severe | Severe | Severe |
| Hypotonia | Yes | Yes | Yes | Yes | Yes |
| Spine | Normal | Kypho-scoliosis | Kyphoscoliosis, Lumbar hyperlordosis | Mild kyphoscoliosis | Gibbus |
| Bone age | Normal | Normal | Normal | Normal | Normal |
| Prominent forehead | Yes | Yes | Yes | Yes | Yes |
| Slant of palpebral fissures | NR | Downslant | Downslant | Upslant | Upslant |
| Proptosis/ Prominent eyes | NR | No | No | Yes | Mild |
| Hypertelorism | NR | Yes | Yes | Yes | Yes |
| Long face | Yes | Yes | Yes | Yes | Yes |
| Prognathism | Yes | Yes | Yes | No | No |
| Ears | NR | Normal | Normal | Low set, large, posteriorly rotated | Low set, large, posteriorly rotated |
| Neuroimaging abnormalities | Megalencephaly, thick corpus callosum, small cerebellum | Normal | Ventriculomegaly, megalencephaly | Shallow orbits, Metopic synostosis, No intracranial abnormality | NR |
| External genitalia | NR | Normal | Normal | Micropenis | Normal |
| Neonatal hypoglycemia | NR | Yes | NR | No | No |
| Seizures | Yes | Yes | NR | No | No |
| High arch palate | NR | Yes | NR | Yes, with palate shelves and gum hypertrophy | Normal |
| Long fingers | Yes | Yes | Yes | Yes | Yes |
| Drooling of saliva | Yes | NR | NR | Yes | Yes |
| Sparse, flared eyebrows | NR | Yes | Yes | Yes | Yes |
- NR, Not recorded; HC, Head circumference.
All clinically reported HERC1 mutations appear to cause a loss of function. The proband reported by Nguyen et al. [2016] was born to consanguineous parents and had a homozygous c.9748C>T (p.Arg3250*) mutation, leading to nonsense mediated mRNA decay and decrease in HERC1 Levels in the skin fibroblasts [Nguyen et al., 2016]. The sibs reported by Ortega-Recalde et al. [2015] were born to non-consanguineous parents and found to be compound heterozygous for c.2625G>A (p.Trp875*), a nonsense mutation and c.13559G>A (p.Gly4520Glu), a putative pathogenic missense mutation. No functional studies were performed, and the missense mutation was presumed to lead to a loss of function by affecting the HECT domain folding. The mutation reported in the sibs here is a splicing variant and leads to production of an abnormally spliced mRNA. This is associated with decreased mRNA abundance, likely due to nonsense mediated decay. Further, this is predicted to result in a truncated protein lacking critical functional domains at the carboxyl end. The loss of the HECT domain is likely of pathogenic significance as this domain interacts with TSC2, and decreases its stability by ubiquitinisation and targeting it for degradation in the absence of TSC1 [Chong-Kopera et al., 2006]. The TSC1–TSC2 complex is a regulator of the mTOR pathway, whose components harbor mutations in patients with macrocephaly and intellectual disability [Mashimo et al., 2009; Mirzaa and Poduri, 2014; Wataya-Kaneda, 2015]. However, the role of HERC1 in this pathway under physiological conditions is unknown, and the protein studies by Nguyen et al. [2016] showed normal TSC2 levels in the patient with HERC1 mutation. Besides the HECT domain, the RLD domains participate in important cellular functions. The RLD2 domain, which is lost in the patients reported in the present study, binds to ARF1 and clathrin, which are important in intracellular membrane trafficking in Golgi and cytoplasmic vesicles [Garcia-Gonzalo and Rosa, 2005].
The tambaleante mouse model of HERC1 has a homozygous p.Gly483Glu mutation, which increases the stability of the protein, hence presumably leading to a gain of function. The mouse phenotype consists of growth restriction, decreased survival, Purkinje cell degeneration in the cerebellum and ataxia [Mashimo et al., 2009]. Impaired neurotransmitter release and dysfunction of the mouse neuromuscular junction has been demonstrated Bachiller et al. [2015]. Features of ataxia and cerebellar hypoplasia were reported in the patient reported by Nguyen et al. [2016]. The human phenotype of mutations in HERC2, a paralog, includes ataxia and intellectual disability, though with other distinctive findings Puffenberger et al. [2012]. The sibs reported by us and by Ortega-Recalde et al. [2015], however, had no cerebellar involvement. It is likely that the nature of mutations as well as effect of modifier genes leads to these phenotypic differences.
To conclude, HERC1 biallelic mutations are likely associated with a phenotype of intellectual disability, macrocephaly, and facial dysmorphism. Further studies and patient reports are required to characterize the phenotypic features, establish genotype–phenotype correlations and ascertain the pathophysiological role of HERC1 in brain development and disease.
ACKNOWLEDGMENTS
Authors are thankful to the family for participation in the study. Department of Biotechnology (DBT), Government of India is also acknowledged for funding support (DBT Project Sanction Order no. BT/PR3193/MED/12/521/2011). We are also thankful to Dr. Vineeth VS and Dr. Usha R. Dutta from Diagnostics Division, Centre for DNA Fingerprinting and Diagnostics, Hyderabad for assisting in RT-qPCR experiment.