Elastins from patients with Williams–Beuren syndrome and healthy individuals differ on the molecular level
Abstract
Williams–Beuren syndrome (WBS) is a congenital disorder, which involves the heterozygous deletion of the elastin gene and other genes on chromosome 7. Clinical symptoms that are associated with hemizygosity of the essential extracellular matrix protein elastin include premature aging of the skin and supravalvular aortic stenosis. However, only little is known about the molecular basis of structural abnormalities in the connective tissue of WBS patients. Therefore, for the first time this study aimed to systematically characterize and compare the structure and amount of elastin present in skin and aortic tissue from WBS patients and healthy individuals. Elastin fibers were isolated from tissue biopsies, and it was found that skin of WBS patients contains significantly less elastin compared to skin of healthy individuals. Scanning electron microscopy and mass spectrometric measurements combined with bioinformatics data analysis were used to investigate the molecular-level structure of elastin. Scanning electron microscopy revealed clear differences between WBS and healthy elastin. With respect to the molecular-level structure, it was found that the proline hydroxylation degree differed between WBS and healthy elastin, while the tropoelastin isoform appeared to be the same. In terms of cross-linking, no differences in the content of the tetrafunctional cross-links desmosine and isodesmosine were found between WBS and healthy elastin. However, principal component analysis revealed differences between enzymatic digests of elastin from healthy probands and WBS patients, which indicates differing susceptibility toward enzymatic cleavage. Overall, the study contributes to a better understanding of the correlation between genotypic and elastin-related phenotypic features of WBS patients. © 2016 Wiley Periodicals, Inc.
Abbreviations
-
- ACN
-
- acetonitrile
-
- ANOVA
-
- analysis of variance
-
- DES
-
- desmosine
-
- HD
-
- hydroxylation degree
-
- HPLC
-
- high performance liquid chromatography
-
- HyP
-
- hydroxyproline
-
- IDES
-
- isodesmosine
-
- MALDI
-
- matrix-assisted laser desorption/ionization
-
- MMP
-
- matrix metalloproteinase
-
- MS
-
- mass spectrometry
-
- PE
-
- pancreatic elastase
-
- SEM
-
- scanning electron microscopy
-
- TE
-
- tropoelastin
-
- TFA
-
- trifluoroacetic acid
-
- TR
-
- trypsin
-
- Tris
-
- 2-amino-2-hydroxymethyl-propane-1,3-diol
-
- WBS
-
- Williams–Beuren syndrome
INTRODUCTION
Williams–Beuren syndrome (WBS; OMIM 194050) is a congenital developmental disorder that is caused by a hemizygous deletion of 1.55–1.84 Mb on the long arm of chromosome 7 [Martens et al., 2008] involving multiple genes including the elastin gene [Pober, 2010]. It occurs at a frequency of about 1 in 7,500 live births [Strømme et al., 2002] and is characterized by a variety of features involving connective tissue and central nervous system abnormalities as well as cardiovascular, craniofacial and behavioral changes as compared to healthy individuals [Gosch and Pankau, 1994; Pankau et al., 1994]. For instance, mild to moderate intellectual deficits, facial dysmorphology, and cardiovascular diseases have been described to occur in WBS patients. Since elastin is an essential extracellular matrix protein present in vertebrate tissues and organs that require elasticity such as blood vessels, skin, and connective tissue [Sage and Gray, 1979], the lack of one elastin gene in WBS patients is associated with severe connective-tissue and cardiovascular pathologies including congenital heart disease, pulmonary stenosis, and supravalvular aortic stenosis [Lowery et al., 1995; Lacolley et al., 2002]. Moreover, a premature skin aging [Ewart et al., 1993], reduced deposition of elastin in dermal elastic fibers and a significantly reduced diameter of these fibers have been described [Dridi et al., 1999; Urbán et al., 2000], which may be connected with increased skin aging that is observed for WBS patients. Overall, however, the molecular-level basis for the development and progression of elastic-tissue pathologies that occur in WBS patients are not well understood yet.
Elastin is produced by various cell types including smooth muscle cells and fibroblasts in the form of its monomeric precursor tropoelastin (TE), which occurs in different isoforms. TE consists of alternating hydrophobic and more hydrophilic K-containing domains, of which the hydrophobic regions are responsible for self-aggregation and tensile properties, while the more hydrophilic domains are involved in cross-linking during formation of mature elastin. Mature elastin contains a variety of unique polyfunctional cross-links including desmosine (DES) and isodesmosine (IDES) that are formed through spontaneous condensation reactions after oxidative deamination of lysine residues by lysyl oxidase or lysyl oxidase-like enzymes. As the core protein of elastic fibers, elastin provides elasticity and resilience to many vertebrate tissues such as aorta, lung, and skin and is, thus, critical for their long-term function [Parks et al., 1993; Mithieux and Weiss, 2005]. In most mammalian tissues, the major part of elastin and elastic fiber formation takes place in a defined developmental window, i.e., starts around the third trimester of gestation in utero, reaches its maximum during early neonatal periods and shortly after begins to be reduced. In mature organs and tissues, elastin synthesis is repressed by post-transcriptional factors. The low turnover of elastin due to its high hydrophobicity, cross-linked structure, and great resistance toward enzymatic cleavage together with the lack of continued elastin production upon maturity reflect the extreme durability and longevity of elastic fibers that reaches the human lifespan of around 74 years [Shapiro et al., 1991; Sivan et al., 2012]. Elastin not only provides mechanical integrity to various tissues, but also plays an important role in the regulation of the cell behavior via bioactive peptides that result from enzymatic degradation of mature elastin by matrix metalloproteinases (MMPs) and neutrophil serine proteases such as human leukocyte elastase, proteinase 3, and cathepsin G [Nagase et al., 2006; Korkmaz et al., 2008]. Studies suggested that in particular elastin-derived peptides containing the GXXPG motif are biologically active, since these are able to interact with the elastin-binding protein a receptor that mediates the action of the peptides [Mecham et al., 1989; Brassart et al., 2001]. Such elastokines for instance promote angiogenesis and influence proliferation, protease activation, and apoptosis [Vrhovski and Weiss, 1998; Maquart et al., 2005; Mithieux and Weiss, 2005; Robinet et al., 2005; Antonicelli et al., 2007]. Damage to elastic fibers together with biological processes triggered by bioactive elastin peptides has been shown to enhance the development and progression of cardiovascular diseases and other pathological conditions. For instance, aortic stenosis is associated with increased activity of MMP-2 and MMP-9 [Helske et al., 2007], atherosclerosis is influenced by MMP-12 [Johnson et al., 2005], and the development of aortic aneurysms is enhanced by MMP-2, MMP-9, and MMP-12 [Curci et al., 1998; Longo et al., 2002]. Moreover, MMP-7 is strongly expressed in tumors of almost every organ in the body and seems to play a vital role in tumor progression and angiogenesis [Nagashima et al., 1997; Wielockx et al., 2004]. Taken together, these examples show that it is of utmost importance to understand and characterize elastin-degrading processes and the nature of the peptides released on degradation.
Since the mechanisms of the development and progression of elastic-tissue pathologies that occur in WBS patients are not well investigated, comparing the molecular-level structures of elastin from healthy donors and WBS patients would help to better understand alterations in the elastic tissue and their functional consequences. Structural investigations on elastin, however, are challenging due to its high hydrophobicity, insolubility, and resistance toward enzymatic cleavage. In recent years, liquid chromatography-mass spectrometry (LC-MS) in combination with bioinformatics methods has become an important tool in protein analytics, which allows the detection and identification of peptides present in enzymatic digests of larger proteins. Using this analytical approach, studies on elastin have been conducted, which focused on its susceptibility to proteolysis by biologically relevant enzymes including MMPs and neutrophil serine proteases [Heinz et al., 2012, 2014, 2011; Schmelzer et al., 2012]. These studies have already provided insight into the molecular-level structure of elastin. The present study deals with the structural characterization of human skin and aortic elastin from WBS patients in comparison with mature human elastin from individuals without WBS. Focus was placed on investigating the morphology of the samples using scanning electron microscopy (SEM). Additionally, the susceptibility of the samples to enzymatic degradation was studied, and the degree of cross-linking was analyzed using mass spectrometric and bioinformatics methods. Methods of statistical analysis including principal component analysis were used to analyze differences between enzymatic digests of elastin derived from healthy individuals and WBS patients.
MATERIALS AND METHODS
Materials
Ten skin biopsies (5 mm in diameter) of individuals of both sexes aged 19–58 years and one skin biopsy of a 90-year-old woman were obtained post-operatively from the tumor-free border of excised skin cancer tissue, respectively. Ten skin samples from WBS patients were obtained as punch biopsies (3 mm diameter) from the forearm of individuals of different sexes and ages between 19 and 46 years after receiving written-informed consent from the parents. Five aortic biopsies from WBS patients (aged 6–39 years) and five aortic punch biopsies of individuals without WBS (aged 40–67 years) were obtained during surgery. An overview on the analyzed tissue samples can be found in Table I. The work on human samples was approved by the ethics committees of the Medical Faculties of the Christian Albrechts University Kiel (Germany) and the Martin Luther University Halle-Wittenberg (Germany), respectively, and performed in compliance with the Helsinki Declaration. All tissue samples were dried in a SpeedVac and weighed before elastin isolation, which was performed as described earlier [Schmelzer et al., 2012]. In brief, tissue samples were subjected to treatment with a variety of reagents including organic solvents, cyanogen bromide, formic acid, mercaptoethanol, and urea (all purchased from Sigma–Aldrich, Steinheim, Germany) to remove all components present in the tissue except for elastin which is resistant to this procedure. All elastin samples were dried in a SpeedVac, weighed and stored at −26 °C before further analysis.
| Tissue | Number of analyzed samples | Ages of individuals upon sample taking/years (mean ± standard deviation) |
|---|---|---|
| Aorta WBS | 5 | 12.2 ± 15.1 |
| Aorta | 5 | 59.8 ± 11.2 |
| Skin WBS | 10 | 38.9 ± 5.9 |
| Skin | 10 | 44.1 ± 12.7 |
| Skin old | 1 | 90 |
Porcine pancreatic elastase (PE) was purchased from Elastin Products Company (Owensville, MO) and sequencing-grade trypsin (TR) from Promega Corporation (Madison, WI). HPLC-grade acetonitrile (VWR Prolabo, Leuven, Belgium) was used. Analytical grade 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris) and formic acid were purchased from Merck (Darmstadt, Germany). Trifluoroacetic acid (TFA) was obtained from Sigma–Aldrich.
Scanning Electron Microscopy
Aortic and skin elastin samples of WBS patients and healthy donors were analyzed by SEM using an environmental scanning electron microscope ESEM XL 30 FEG (Philips, Amsterdam, the Netherlands) as described previously [Heinz et al., 2013]. Overall, five aortic elastin samples and six skin elastin samples from individuals without WBS, respectively, and two aortic elastin samples and six skin elastin samples from WBS patients, respectively, were investigated.
Proteolysis of Human Elastin
Aortic and skin elastin samples were dispersed in 50 mM Tris buffer, pH 7.5, at a concentration of 1 mg ml−1, respectively. All samples were digested with PE and TR for 24 hr at 37 °C using enzyme-to-substrate ratios of 1:100 (w/w), respectively. All digestions were stopped by adding TFA to a final concentration of 0.5% (V/V), and the samples were stored at −26 °C prior to further analysis.
Mass Spectrometric Analysis of Peptide Digests
Analysis of the enzymatic digests of all elastin samples was carried out by nanoHPLC/MALDI-TOF/TOF MS using an UltiMate 3000 RSLCnano system (Thermo Fisher, Idstein, Germany), a Probot microfraction collector (Thermo Fisher), and a 4800 MALDI-TOF/TOF Analyzer (AB Sciex) as described previously [Heinz et al., 2013]. Digests of all elastin samples were furthermore analyzed by nanoHPLC-nanoESI-QqTOF MS(/MS) using an UltiMate 3000 nanoHPLC system (Thermo Fisher) coupled to a QqTOF mass spectrometer Q-TOF-2 (Waters/Micromass, Manchester, UK) as described earlier [Heinz et al., 2014]. Chromatographic separation of the peptides was performed at 40 °C using a flow rate of 300 nl min−1 according to a protocol published earlier [Heinz et al., 2014]. The operating conditions for the mass spectrometer during MS/MS measurements were chosen as described earlier [Heinz et al., 2010] except for the sample cone voltage, which was lowered to 20 V to minimize in-source decay. Full scan MS measurements were performed for five healthy skin and five WBS skin elastin samples. The samples were measured in random order, and each sample was measured four times. Data were acquired in the m/z range between 40 and 1,550.
Peptide Sequencing
Tandem mass spectra obtained from MALDI-TOF/TOF experiments were processed into de-isotoped peak lists with Mascot Distiller (Matrix Science, London, UK). Automated de novo sequencing of the nanoESI-QqTOF MS/MS and MALDI-TOF⁄TOF MS/MS data followed by database matching was performed using the software Peaks Studio (version 7; Bioinformatics Solutions, Waterloo, Canada) [Zhang et al., 2012]. The searches were taxonomically restricted to Homo sapiens and the enzyme was set to “none.” The formation of hydroxylated proline residues was considered as variable modification. For the MALDI TOF/TOF MS/MS data, mass error tolerances for precursor and fragment ions were set to 50 ppm and 0.3 Da, respectively, and for the QqTOF MS/MS data, mass tolerances for precursor and fragment ions were set to 40 ppm and 0.1 Da, respectively. For statistical analysis of MS data using the Progenesis QI software (Nonlinear Dynamics, Newcastle upon Tyne, UK), nanoESI QqTOF MS/MS data were imported into the software and converted into a single mgf. The mgf was submitted to a local Mascot server (Matrix Science, London, UK) and peptides were sequenced using the settings described above.
Detection and Quantification of Desmosine and Isodesmosine
All elastin samples were treated as described previously [Heinz et al., 2014]. In brief, LC-MS analysis of DES and IDES was carried out using an Agilent 1100 LC system (Agilent, Waldbronn, Germany) coupled to a quadrupole ion trap mass spectrometer Finnigan LCQ (Thermo Fisher, San Jose, CA) via an electrospray interface using the settings described earlier [Heinz et al., 2014].
Semi-Quantitative Determination of Hydroxyproline Content
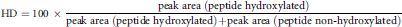
Two singly hydroxylated peptides and their non-hydroxylated counterparts that occurred in the mass spectra with high abundance were chosen for semi-quantitative analysis. The sequences of the two peptide pairs were AGIPGVGPF (813.44 Da)/AGI(HyP)GVGPF (829.43 Da) and GGVGIPGGVVGA (938.52 Da)/GGVGI(HyP)GGVVGA (954.51 Da). In case different charge states of one peptide species occurred, peak areas of all charge states were taken into account and added up.
Statistical Analysis of Full Scan MS Data
Full scan MS raw data were imported into Progenesis QI software for statistical analysis. After normalization and automatic alignment of the MS data, statistical analysis was performed on all detected features using transformed normalized abundances by one-way analysis of variance (ANOVA). All peptides with Mascot score >18 and P ≤ 0.05 were included and a >2-fold change and coefficient of variation <50% were regarded as significant. Principal component analysis (PCA) was carried out using Progenesis QI software based on the normalized abundances of identified elastin peptides.
RESULTS AND DISCUSSION
Elastin Content of Tissues and Ultrastructure of Elastin Fibers
All tissue samples of WBS patients and healthy donors were weighed before and after isolation of elastin to determine the amount of elastin content of healthy and diseased tissue. It was found that WBS skin contains significantly less elastin (1.6 % ± 0.4%) compared to skin elastin from healthy probands (6.0% ± 2.4%) (Fig. 1A). For aortic samples, slightly less elastin was identified in the WBS tissue samples (20.1% ± 2.5%) as compared to aortic samples from individuals without WBS (23.3% ± 0.4%), however, this was not found to be statistically significant (Fig. 1A). In addition to the elastin content of the tissue samples, the ultrastructure of the isolated elastin fibers was investigated by SEM. From all samples that were analyzed by SEM (19 samples in total), representative images displaying characteristic features were chosen and are summarized in Fig. 2. For aortic elastin significant differences between samples of healthy donors and WBS patients were found (Fig. 2A–D). Elastin fibers from healthy donors appeared intact, showed smooth surfaces, and were organized mainly in larger fiber bundles with diameters of about 1–1.5 μm, while they contained only few smaller fibers with diameters of about 100 nm (Fig. 2A and B). In contrast, elastin fibers from WBS patients showed predominantly rough surfaces, were often broken, and larger fibers bundles appeared disintegrated resulting in much smaller elastin fibers with diameters below 1 μm (Fig. 2C and D). Similar results were obtained for skin elastin (Fig. 2E–J). Skin elastin fibers from young individuals (around 35 years) showed smooth surfaces, appeared intact, and consisted mainly of larger fiber bundles of diameters above 1 μm (Fig. 2E and F). Skin elastin fibers of WBS patients, however, were fragmented and damaged, and larger fiber bundles appeared disintegrated into smaller fibers with diameters below 1 μm (Fig. 2G and H). The results obtained for elastin fibers of healthy, old individuals (around 90 years) were similar in that the fibers were broken and fragmented (Fig. 2I and J). However, unlike in the samples of WBS patients, larger fiber bundles were not completely disintegrated. Although many smaller fibers of diameters below 1 μm were clearly visible, they were still organized in larger bundles.
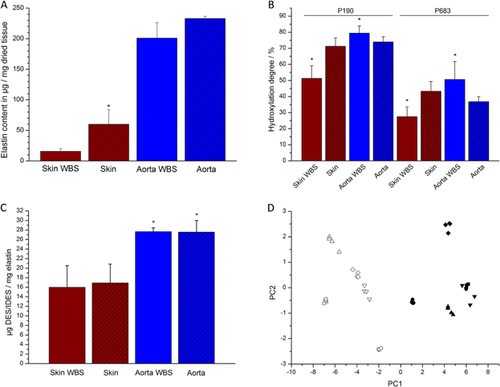
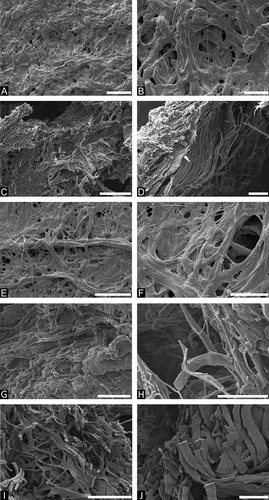
In summary, skin of WBS patients was found to contain low amounts of elastin and fragmented and disintegrated elastin fibers, whereas skin of healthy individuals contained higher amounts of elastin and showed elastin fibers with smooth surfaces. These findings are in agreement with the results obtained in previous studies [Dridi et al., 1999; Urbán et al., 2000; Kozel et al., 2014]. For instance, transmission electron micrographs revealed reduced elastin deposition in skin of WBS patients [Urbán et al., 2000]. Another study found rarefied and dispersed elastic fibers in skin samples of WBS patients as compared to healthy individuals by fluorescence and standard optical microscopies [Dridi et al., 1999]. Both studies are consistent with the smaller amounts of elastin in WBS skin identified in this study as compared to healthy individuals. Furthermore, a recent study described a reduced viscoelasticity and a decreased elastic modulus of skin of WBS patients as compared to healthy subjects [Kozel et al., 2014], which is also likely to be related to the smaller amounts of damaged elastin fibers found in this work. Overall, these phenotypic features are strongly connected to the hemizygosity of the elastin gene in WBS patients.
The results obtained for aortic elastin are also in agreement with previous studies [O'Connor et al., 1985; Li et al., 1998; Lacolley et al., 2002; Urbán et al., 2002; Unuma et al., 2011]. In a case study on a WBS patient with segmental stenosis in large arteries, the authors found that the stenoses were associated with disrupted, fragmented elastic lamellae [Unuma et al., 2011]. Moreover, previous work on carotid arteries and a renal artery of WBS patients also revealed a reticular, fragmented appearance of elastic fibers in the media of WBS patients by electron microscopic investigations [Lacolley et al., 2002]. Additionally, an increased distensibility, an arterial wall hypertrophy, and lower elastic modulus of the arteries were observed. These findings are consistent with the results of this study that revealed the presence of disintegrated and damaged elastin fibers in WBS aorta. Another study, which compared elastogenesis and proliferation rates of cultured aortic smooth muscle cells and skin fibroblasts of WBS patients, patients with isolated supravalvular aortic stenosis and healthy control subjects, described significantly lower elastin mRNA levels, and consequently deposition of lower amounts of insoluble elastin for WBS cells in vitro, which coincided with an increase in the proliferation rate of the cells [Urbán et al., 2002]. The authors discussed these results in the context of in vivo findings in aorta, pulmonary artery, and extramural coronary artery samples and concluded that the deposition of lower amounts of elastin results in the formation of thinner elastic lamellae, however, also an increase in proliferation of smooth muscle cells and, hence, hypertrophy of the artery walls, which may eventually lead to segmental arterial occlusion [Urbán et al., 2002]. Similar results were obtained in a study on ELN ± mice which was developed as a WBS mouse model. It was found that ELN ± mice revealed a 47% decrease in elastin mRNA compared to ELN +/+ mice at birth resulting in the formation of thinner elastic lamellae [Li et al., 1998]. However, at the same time a compensatory increase in the number of elastic lamellae was observed in the aorta, which was found to maintain arterial extensibility [Li et al., 1998]. The current study also showed a slightly lower elastin content of WBS aortic tissue as compared to aortic tissue from individuals without WBS; however, this difference was not as pronounced as in the case of skin elastin. This may be related to the overall higher age of the aortic samples from individuals without WBS, which are likely to have undergone changes due to tissue aging, whereas the samples from WBS patients are almost not affected by tissue aging. This is supported by results obtained in a study on aortic punch biopsies similar to the ones used in this work, which revealed a decrease of the amount of interlaminar elastin and a decrease of tropoelastin mRNA levels with increased age, respectively [Fritze et al., 2011]. The similarities in the amounts of elastin found between aortic samples from WBS patients and healthy subjects may also be associated with an increase of the number of the elastic lamellae [O'Connor et al., 1985; Li et al., 1998], which may partly compensate for the lower amounts of elastin that are produced. For ethical reasons, it was not possible to obtain aortic samples from younger healthy individuals for comparison with aortic elastin from WBS patients.
Susceptibility Towards Enzymatic Cleavage and Analysis of Molecular-Level Structure
All samples were well cleaved by PE (Fig. 3), which results from the fact that PE is an aggressive elastase with a broad cleavage specificity (cleaves predominantly C-terminal to A, V, L, and I, but also C-terminal to Y, F, K, P, R, and G) [Powers et al., 1977; McRae et al., 1980]. With respect to the presence or absence of certain domains, MS analysis of the enzymatic elastin digests revealed that exons 24A and 26A are spliced out from skin and aortic elastin of both WBS patients and healthy individuals. For instance, peptides were detected containing residues from domains 26 and 27 as well as 23 and 24, which indicate that domains encoded by exons 24A and 26A are not present. The data furthermore points to the absence of domain 22 as no linear peptides from this domain were identified in any of the enzymatic digests. These results are in agreement with previous studies on human skin and aortic elastin [Getie et al., 2005; Schmelzer et al., 2005; Taddese et al., 2010; Heinz et al., 2012, 2014, 2011] and suggest that TE isoform 2 is present in both healthy individuals and WBS patients.

Overall, comparable sequence coverages of 71% (64%) and 69% (79%), based on TE isoform 2, were obtained upon sequencing of linear peptides from enzymatic digests of skin (aortic) elastin from WBS patients and skin elastin from healthy donors, respectively (Fig. 3). The identified peptides are all derived from non-cross-linked regions of elastin, while the overall lack of identified peptides from the cross-linked KA and KP domains can be attributed to the fact that cross-linked elastin peptides still cannot be sequenced with commercially available bioinformatics tools. It is worth mentioning that >75% (>60%) of the cleavage sites found in skin (aortic) elastin of WBS patients and skin (aortic) elastin of healthy donors were identical. Obviously, this is mainly related to the broad cleavage specificity of PE. Moreover, these results indicate that elastin from healthy donors and WBS patients are structurally similar, including the fact that K residues seem to be predominantly involved in cross-links as otherwise linear peptides from KA and KP domains would have been identified. This assumption is supported by another experiment that was carried out using TR, which cleaves C-terminal to K and R residues. The results were consistent with earlier studies which reported virtually no elastinolytic activity for TR [Thomas and Partridge, 1960; Heinz et al., 2014]. Overall, it was found that TR neither cleaves elastin from WBS patients nor elastin from healthy individuals, which indicates that the K residues are mostly involved in cross-linking.
Proline Hydroxylation and Elastin Cross-Linking
Proline hydroxylation is an important post-translational modification of elastin and occurs as a partial modification, i.e., the same P residue is hydroxylated or non-hydroxylated in different TE molecules [Uitto, 1979]. In this study, 15 hydroxylated proline (HyP) residues were identified in skin elastin from healthy individuals (Fig. 3A). With the exception of P347, these hydroxylation sites were also detected for WBS elastin together with two additional sites (P387 and P595). Moreover, 20 partially hydroxylated sites were detected in aortic elastin from healthy individuals (Fig. 3B). With the exception of P147, P337, P347, P387, P613, and P726, 14 of these sites were also identified for aortic elastin from WBS patients. The lower number of hydroxylation sites identified in aortic elastin from WBS patients may be related to the fact that potential hydroxylation sites are not or less hydroxylated in WBS elastin. Moreover, less peptides were identified for these samples during MS analysis. Overall, these results suggest that same sites are hydroxylated in elastin from healthy individuals and WBS patients, which also supports the assumption that both elastins are similar on the molecular level. In addition to identifying P hydroxylation sites, the hydroxylation degree (HD) was determined for two P residues. Two abundant peptides that occur both in hydroxylated and non-hydroxylated form were used for analysis (Fig. 3A), one of which contained P190 and the other one P683 (based on numbering of TE, isoform 9). Overall, it was found that both P residues are less hydroxylated in skin elastin from WBS patients as compared to elastin from healthy probands (Fig. 1B). The HD of P190 was 71.2% ± 5.2% for healthy skin elastin, whereas it was only 51.3% ± 7.9% in WBS skin elastin. With respect to P683, HDs of 43.3% ± 6.1% and 27.4% ± 6.1% were obtained for skin elastin from healthy probands and WBS patients, respectively. Interestingly, for the aortic samples the opposite was found (Fig. 1B). The two residues were significantly stronger hydroxylated in elastin from WBS patients. The HDs of P190 were 73.9% ± 3.2% and 79.4% ± 4.5% in elastin of healthy probands and WBS patients, respectively. For P683, 36.8% ± 3.1% and 50.6% ± 11.2% were determined for healthy probands and WBS patients, respectively. One can only speculate about the role of hydroxyproline (HyP) in elastin. Several reports suggest that HyP does not interfere with oxidative deamination of K residues [Narayanan et al., 1978] and is not required for synthesis and secretion of elastin, however, seems to influence the coacervation temperature and, thus, the formation of elastic fibers [Rosenbloom and Cywinski, 1976; Uitto, 1979; Urry et al., 1979]. A recent study on hydroxylated elastin peptides, for instance, showed that the presence of HyP increases the coacervation temperature and alters the self-assembly process, which in turn may have consequences on the ability of TE to cross-link and form mature elastin [Bochicchio et al., 2013].
Parts of all samples were not digested enzymatically but were used for analysis of the DES and IDES content (Fig. 1C). No significant differences were found between samples from the same type of tissue regardless of whether the samples were obtained from healthy probands or WBS patients (skin from healthy individuals 16.9 ± 3.9 μg DES and IDES per mg elastin; skin from WBS patients 16.0 ± 4.5 μg DES and IDES per mg elastin; aorta from healthy individuals 27.5 ± 2.5 μg DES and IDES per mg elastin; aortic elastin from WBS patients 27.6 ± 0.8 μg DES and IDES per mg elastin). Interestingly, aortic elastin from both healthy individuals and WBS patients contained significantly more DES and IDES than skin elastin. This indicates that the cross-linking pattern of elastin from skin and aorta is different, which in turn may have something to do with the architecture and function of elastin in these tissues. While aortic tissue contains about 28–32% of elastin (based on dry weight of tissue) in parallel elastin fiber lamellae, skin contains only about 0.6% of elastin in randomly oriented, loose fibers [Uitto, 1979]. The more compact architecture of aortic elastin may require a different cross-linking pattern.
Statistical Comparison of Elastin From Healthy Individuals and WBS Patients
PE digests of five skin elastin samples from healthy probands and five skin elastin samples from WBS patients were subjected to statistical analysis by ANOVA and PCA. The PCA scores plot showed clear separation of the WBS elastin and the healthy elastin based on the first principal component, which described more than 82% of the variation in the data set (Fig. 1D). This indicates that there are significant differences between elastin from WBS patients and healthy individuals. Indeed, a total of 27 peptides were identified that significantly varied in their normalized abundances between skin elastin from healthy probands and WBS patients (Table II). Twelve of these peptides showed higher abundances in elastin digests of WBS patients, and it is interesting to note that eight of these originate from the C-terminal TE domains 26–29. This may suggest that elastin from WBS patients is slightly more susceptible toward enzymatic cleavage in its C-terminal region, which in turn may be a result of structural differences as compared to elastin from healthy probands.
| Peptide sequence | Monoisotopic mass/Da | Ratio normalized abundances WBS/adult skin | Residues (based on TE isoform 2) | Domain |
|---|---|---|---|---|
| GAGLGGV(HyP)GVGGL | 1025.54 | 0.5 | 109–121 | 7 |
| VLPGVPT | 681.40 | 0.5 | 165–171 | 10 |
| GIPGVGPF | 742.39 | 2.5 | 188–195 | 11/12 |
| VGPFGGPQPGVPL | 1220.64 | 2.1 | 192–204 | 12 |
| GVLPGVGGA | 725.40 | 2.1 | 272–280 | 16 |
| GVLPGVGGAGV(HyP)GVPG | 1304.69 | 0.4 | 272–287 | 16 |
| AIPGIG | 526.31 | 0.4 | 288–293 | 16 |
| AIPGIGG | 583.33 | 0.3 | 288–294 | 16 |
| AIPGIGGI | 696.41 | 0.5 | 288–295 | 16 |
| AIPGIGGIA | 767.45 | 0.4 | 288–296 | 16 |
| GLVPGGPGFGPG | 1010.51 | 0.4 | 321–332 | 18 |
| G(HyP)GVVGVPGAGVPG | 1134.59 | 0.3 | 330–343 | 18 |
| AGIPVVPGAGI(HyP)G | 1119.62 | 0.4 | 349–361 | 18 |
| GIPVVPG | 637.38 | 0.3 | 350–356 | 18 |
| GIPVVPGAGI(HyP)G | 1048.58 | 0.3 | 350–361 | 18 |
| GGFPGFGVGVGGIPG | 1273.63 | 3.4 | 402–416 | 20 |
| GVGVGGI(HyP)GVA | 897.48 | 0.4 | 408–418 | 20 |
| VGLAPGVGVAPG | 992.55 | 0.4 | 495–506 | 24 |
| GAGIPGLGVGV | 895.50 | 2.2 | 547–557 | 26 |
| GI(HyP)GLGV | 627.36 | 0.4 | 549–555 | 26 |
| GIPGLGVGV | 767.44 | 2.0 | 549–557 | 26 |
| GVGVPGLGVGA | 881.48 | 3.0 | 556–566 | 26 |
| GVPGLGVGA | 725.39 | 2.2 | 558–566 | 26 |
| GAGVPGFGAVPG | 984.50 | 2.4 | 574–584 | 26/27 |
| GALGGVGIPGGVVGA | 1179.66 | 2.1 | 607–621 | 28/29 |
| LGGVGIPGGVVGA | 1051.59 | 2.1 | 609–621 | 28/29 |
| GGVGIPGGVVGA | 938.52 | 2.6 | 610–621 | 28/29 |
- Hydroxylated proline residues are denoted as HyP. Potentially bioactive sequences are highlighted in bold.
Moreover, it is worth mentioning that all seven HyP-containing peptides that were identified occurred with higher abundances in elastin from healthy probands (Table I). This may have something to do with the overall lower HD of skin elastin from WBS patients, which leads to an overall lower number of HyP-containing peptides. With respect to the release of potentially bioactive peptides, it was found that 9 of the 27 peptides that showed significant differences in their normalized abundances between the samples of WBS patients and healthy individuals contained bioactive motifs (Table II). For instance, VGVPG and GLVPG have been described to be chemotactic to monocytes [Bisaccia et al., 1994; Castiglione Morelli et al., 1997], while GAVPG has been found to induce the pro-MMP-2 secretion [Heinz et al., 2010]. Moreover, GVLPG, GFGPG, and GLVPG are likely to stimulate the pro-MMP-1 secretion [Heinz et al., 2012], and VGVAPG displays a variety of bioactivies as it strongly interacts with the elastin-binding protein [Mecham et al., 1989]. These activities include, for instance, chemotaxis of monocytes and fibroblasts [Senior et al., 1984], induction of the expression of pro-MMP-1 in fibroblasts [Brassart et al., 2001] and stimulation of the proliferation of smooth muscle cells [Mochizuki et al., 2002]. No biological activities have been reported for GGFPG, GPQPG, GLAPG, and GGIPG yet although in particular GLAPG and GPQPG adopt the type VIII beta turn conformation which has been hypothesized to facilitate the interaction of the peptide with the elastin-binding protein [Moroy et al., 2005]. When comparing the nine peptides with bioactive motifs identified in this study, it becomes obvious that in WBS samples only the abundance of a peptide containing GAVPG is significantly increased as compared to the samples from healthy individuals (Table II). As GAVPG has been described to stimulate the production of MMP-2, the action of GAVPG may accelerate elastin degradation in WBS patients should it be released by biologically relevant enzymes such as MMPs and neutrophil serine proteases. However, it needs to be mentioned that the results in terms of bioactive peptides do not point to a clear role of such peptides in the enhanced degradation of elastin fibers in WBS patients as other biologically active motifs have been found to be released in higher abundance from elastin of healthy individuals (Table II). Further experiments with a range of enzymes will, thus, be carried out to obtain a deeper understanding of the structural differences between elastin from WBS patients and healthy individuals. Moreover, the analysis of other typical elastin cross-links will allow insights into the exact cross-linking pattern of elastin from healthy individuals and WBS patients. Furthermore, other proteins that are required for elastogenesis will be investigated to find out whether an altered elastogenesis has an impact on the structure of elastin fibers from WBS patients.
ACKNOWLEDGMENTS
The work was supported by the German Research Foundation (DFG) grants HE 6190/1-1 and HE 6190/1-2 (A.H.), by the European Regional Development Fund of the European Commission (C.U.S.), and by the Departamento Administrativo de Ciencia, Tecnología e Innovación—Colciencias (Colombia) and the Universidad Nacional de Colombia (Bogotá D.C., Colombia) (A.C.M.H.). Dr. Frank Heyroth (Interdisciplinary Center for Materials Science, Martin Luther University Halle-Wittenberg, Germany) is thanked for assistance with scanning electron microscopy and Dr. Wolfgang Hoehenwarter (Leibniz Institute for Plant Biochemistry, Halle, Germany) for help with the statistical analysis. Prof. Ulrich Stock (University Hospital Frankfurt, Germany) and Prof. Johannes Wohlrab (Department of Dermatology and Venereology, Martin Luther University Halle-Wittenberg, Germany) are thanked for providing aortic punch biopsies and skin biopsies, respectively. Moreover, the authors would like to thank the WBS patients and their families for their willingness to support the study.