A mutation in COL4A2 causes autosomal dominant porencephaly with cataracts
Abstract
Mutations in COL4A1 are well described and result in brain abnormalities manifesting with severe neurological deficits including cerebral palsy, intellectual disability, and focal epilepsy. Families with mutations in COL4A2 are now emerging with a similar phenotype. We describe a family with an autosomal dominant disorder comprising porencephaly, focal epilepsy, and lens opacities, which was negative for mutations in COL4A1. Using whole exome sequencing of three affected individuals from three generations, we identified a rare variant in COL4A2. This COL4A2 (c.2399G>A, p.G800E, CCDS41907.1) variant was predicted to be damaging by multiple bioinformatics tools and affects an invariable glycine residue that is essential for the formation of collagen IV heterotrimers. The cataracts identified in this family expand the phenotypic spectrum associated with mutations in COL4A2 and highlight the increasing overlap with phenotypes associated with COL4A1 mutations. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Collagen IV (COL IV), an important component of the basal lamina, is comprised of six α-polypeptide chains encoded by six differentially expressed genes [Schmidt et al., 1993]. These genes are arranged in pairs on chromosomes 2, 13, and X, and each pair produces two genetically identical paralogous α-peptides through a bidirectional promoter [Haniel et al., 1995; Khoshnoodi et al., 2008]. The COL IV α-peptides assemble into heterotrimers with the following stoichiometries: α1α1α2, α3α4α5, and α5α5α6 [Khoshnoodi et al., 2008]. Aberrations to COL IV heterotrimers can lead to genetic and acquired diseases [Lemmink et al., 1997] (Supplementary Table SI). COL4A1 and COL4A2 are the most abundantly and ubiquitously expressed COL IV peptides [Favor et al., 2007]. Mutations in COL4A1 and COL4A2 can affect multiple organs resulting in various cerebral, ocular, renal and muscular pathologies [Favor et al., 2007; Kuo et al., 2014].
Mutations in COL4A1 result in well-recognized phenotypes (Supplementary Table SI). The current clinical picture of COL4A2 is limited to porencephaly type II (MIM 614483) and intracerebral haemorrhage (MIM 614519). While systemic and eye pathologies have been reported in isolated patients, these clinical features are less well recognized as part of the COL4A2 spectrum (Supplementary Table SII) [Verbeek et al., 2012; Gunda et al., 2014].
Cerebral defects are prominent in all patients with COL4A2 mutations and there is in vivo evidence from mice model studies that missense mutations in Col4a2 can also induce ocular, myopathic, and systemic defects [Favor et al., 2007; Kuo et al., 2014]. We expand the clinical spectrum of COL4A2 by identifying a novel missense mutation in a family with autosomal dominant inheritance of porencephaly, focal epilepsy and lens opacities.
MATERIALS AND METHODS
Informed written consent was obtained from all subjects or their parents or legal guardians in the case of minors. The study was approved by the New Zealand Health and Disability Ethics Committee and the Adelaide Women's and Children's Health Network Human Research Ethics Committee.
Exome Sequencing
Whole exome sequencing (Roche Nimblegen SeqCap v3) was performed on a HiSeq2500 (Illumina) by the South Australian Cancer Council Genomics Facility on three affected individuals (I-2, II-2, III-1; Fig. 1A). Reads were mapped to the human genome (hg19) using BWA-MEM [Li and Durbin, 2009] and mapping refined using Genome Analysis Toolkit (GATK) version 3.2-2 [DePristo et al., 2011]. Mapping achieved a minimum median target coverage depth of 46 reads/sample and covered 95% of intended targets with at least 10 reads (Supplementary Fig. S1). Single nucleotide variants and small insertions and deletions were called by the GATK haplotype caller version 3.2-2 [DePristo et al., 2011]. Larger copy number and structural variants were analysed by CoNIFER [Krumm et al., 2012]; however, no segregating, rare copy number variants were detected. All variants were annotated for allele frequency, clinical significance, locus identity, and likely pathogenicity using ANNOVAR [Wang et al., 2010]. Novel genotypes shared between affected individuals, but absent from a control set of 15 exomes, were separated using the vcf-contrast module from VCFtools [Danecek et al., 2011].
RESULTS
Clinical Reports
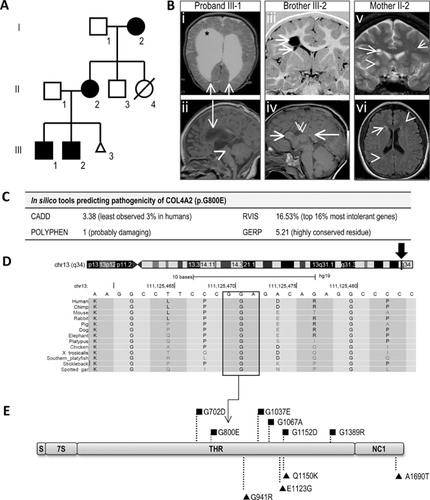
The proband (Fig. 1A, III-1) was a 16-year-old male with spastic quadriplegia and apparent intellectual disability who was non-verbal and fully dependent. First concerns arose at 11 weeks due to poor head control with seizure onset at 2 years. He developed intractable multiple types of focal seizures and status epilepticus. Non-progressive bilateral small posterior lens opacities were found at 3 years. EEGs showed left hemisphere slowing and right parietal epileptiform activity. MRI brain showed right frontal communicating porencephaly with dilated lateral ventricles (Fig. 1B, i) and destruction of the right caudate nucleus, lentiform nucleus, internal capsule, thalamus, and right hippocampus (Fig. 1B, ii).
The proband's 13-year-old brother (III-2) had developmental delay with walking at 18 months and first words at 2 years. Autism spectrum disorder was diagnosed at 5 years. He had apparent intellectual impairment and could read simple sentences and do basic addition. He had focal dyscognitive seizures from 3 to 11 years. At 14 months, he had non-progressive bilateral posterior subscapular lens opacities. His neurological examination was normal. EEG at three years was normal. MRI revealed a right frontal porencephalic cyst communicating with the right lateral ventricle (Fig. 1B, iii). There was partial destruction of the periventricular white matter and caudate body. A smaller cyst was present in the right external capsule. The corpus callosum had an unusual appearance with thickened genu and splenium and absence of the intervening body (Fig. 1B, iv). COL4A1 sequencing was normal.
The 41-year-old mother (II-2) was asymptomatic and of apparent normal intellect. She had a mild left internuclear ophthalmoplegia. MRI showed a cluster of small cysts in the right frontal periventricular white matter and bilateral anterior caudate nuclei (Fig. 1B, v). There were numerous scattered T2/FLAIR hyperintense lesions in keeping with foci of gliosis, scattered throughout the white matter but prominent frontally (Fig. 1B, vi).
The 69-year-old maternal grandmother (I-2) had a mild right hemiplegia noted in the first year of life. She had learning difficulties at school but was of apparent normal intellect in adult life. She developed focal dyscognitive seizures in childhood and convulsive seizures at 47 years. She continued to have intractable focal seizures with episodes of status epilepticus. On examination, she has an obvious right hemiplegia. She refused neuroimaging and an EEG. She developed bilateral cataracts at 62 years of age.
Exome Analysis
There were 8,388 novel genotypes that were shared between the three affected individuals (Supplementary Table SIII). Of these, 409 variants passed our frequency and read quality criteria (Supplementary Table SIII). Only one out of 69 coding variants passed our filtering criteria and segregated with the clinical phenotype: COL4A2 (c.2399G>A, p.G800E, CCDS41907.1; Supplementary Table SIV). Sanger sequencing confirmed the segregation of the variant in the proband, his brother, mother and grandmother. (Supplementary Fig. S2F). The COL4A2 p.G800E variant was predicted as likely disease causing by multiple bioinformatics tools (Fig. 1C). The glycine at position p.800 is invariable, from primates through to fish (Fig. 1D).
DISCUSSION
Lens opacities have not been recorded in previous COL4A2 reports but are well recognized in COL4A1-related disorders, together with other ocular defects [Coupry et al., 2010]. To date, only one COL4A2 mutation has been associated with ocular defects that included myopia, amblyopia, and abnormal optic discs (Supplementary Table SII) [Verbeek et al., 2012]. Lens opacities in humans have not been described and are noted here in young children with the COL4A2 mutation; in contrast, the grandmother's cataracts may be an unrelated incidental age-related finding.
The phenotypes observed in human COL4A2-related disorders are also found in mice carrying heterozygous Col4a2 mutations (Supplementary Table SII) [Favor et al., 2007; Kuo et al., 2014]. The severity and penetrance of eye, brain, and muscular defects varies markedly among inbred (C57B1/6J) mice with the same Col4a2 mutations [Kuo et al., 2014]. In humans, dominant COL4A2 mutations also exhibit incomplete penetrance (Supplementary Table SII) [Verbeek et al., 2012; Yoneda et al., 2012].
The majority of COL4A1 and COL4A2 mutations occur at highly conserved glycine residues within the triple helical region (THR; Fig. 1D) [Favor et al., 2007]. These THR glycine mutations likely destabilize COL IV heterotrimer formation leading to the accumulation of a misfolded protein and subsequent chronic stress on the endoplasmic reticulum [Yoneda et al., 2012; Kuo et al., 2014]. In both mice and humans, COL4A2 THR glycine mutations usually lead to severe, early onset cerebral pathologies but with a better prognosis compared to similar mutations in COL4A1 (Supplementary Table SII) [Favor et al., 2007]. It has been hypothesized that the difference in phenotypic severity of COL4A1 versus COL4A2 mutations is due to the stoichiometry of α1:α1:α2 per heterotrimer [Favor et al., 2007; Khoshnoodi et al., 2008; Jeanne et al., 2012]. A heterozygous COL4A2 mutation will lead to 50% of COL4A1/A2 trimers with a mutant peptide, which will be less than a heterozygous COL4A1 mutation with up to 75% of COL4A1/A2 trimers having a mutant peptide [Favor et al., 2007; Jeanne et al., 2012]. The location of the pathogenic variant relative to different functional domains of the COL4A1/A2 peptides also influences the clinical outcome [Des Parkin et al., 2011]. Non-glycine mutations (NGMs) within the THR and also the non-collagenous domain are likely low penetrance, risk alleles for late onset intracranial haemorrhage with good survival; a distinctly different phenotype to that caused by glycine mutations (Supplementary Table SV) [Jeanne et al., 2012]. Our review of COL4A2 variations showed that all three previously reported NGMs [Jeanne et al., 2012] are present in the ExAC database while pathogenic THR glycine mutations are absent (Fig. 1E, Supplementary Table SVI). Nonetheless, some NGMs decrease the ratio of extracellular to intracellular COL4A2 relative to wild-type and also two of three variants induced stress in the endoplasmic reticulum in a similar way to THR glycine mutations, which supports their pathogenicity [Jeanne et al., 2012].
In conclusion, we identified a novel COL4A2 (c.2399G>A, p.G800E, CCDS41907.1) mutation in an autosomal dominant family with porencephaly and ocular abnormalities. The mutation is predicted to be pathogenic based on the substitution of a highly conserved, critical THR-glycine residue and segregation with the phenotype. We extend the COL4A2 phenotype spectrum, with the observation of lens opacities in some affected individuals, thus reducing the distinction between COL4A1 and COL4A2 mutations. This highlights the importance of simultaneous screening of both COL4A1 and COL4A2 genes for accurate diagnosis of early onset porencephaly and further ophthalmological management where indicated.
ACKNOWLEDGMENTS
We wish to thank the family for participating in our research, Alison Gardner for support with bioinformatics, eResearchSA, and the SA Cancer Council Genomics Facility. This project was supported by: NH&MRC grant 628952 to IES and JG, Senior Research Fellowship 1041920 (JG) and Practitioner Fellowship 1006110 (IES); WCH foundation MS McLeod research fellowship (MC); Health Research Council of New Zealand project grant 10/402 and CureKids New Zealand (LGS, IES, and SJP).