Genotype–phenotype characterization in 13 individuals with chromosome Xp11.22 duplications
Abstract
We report 13 new individuals with duplications in Xp11.22-p11.23. The index family has one male and two female members in three generations with mild-severe intellectual disability (ID), speech delay, dysmorphic features, early puberty, constipation, and/or hand and foot abnormalities. Affected individuals were found to have two small duplications in Xp11.22 at nucleotide position (hg19) 50,112,063–50,456,458 bp (distal) and 53,160,114–53,713,154 bp (proximal). Collectively, these two regions include 14 RefSeq genes, prompting collection of a larger cohort of patients, in an attempt to delineate critical genes associated with the observed phenotype. In total, we have collected data on nine individuals with duplications overlapping the distal duplication region containing SHROOM4 and DGKK and eight individuals overlapping the proximal region including HUWE1. Duplications of HUWE1 have been previously associated with non-syndromic ID. Our data, with previously published reports, suggest that duplications involving SHROOM4 and DGKK may represent a new syndromic X-linked ID critical region associated with mild to severe ID, speech delay +/− dysarthria, attention deficit disorder, precocious puberty, constipation, and motor delay. We frequently observed foot abnormalities, 5th finger clinodactyly, tapering fingers, constipation, and exercise intolerance in patients with duplications of these two genes. Regarding duplications including the proximal region, our observations agree with previous studies, which have found associations with intellectual disability. In addition, expressive language delay, failure to thrive, motor delay, and 5th finger clinodactyly were also frequently observed in patients with the proximal duplication. © 2015 Wiley Periodicals, Inc.
Abbreviations
-
- ID
-
- intellectual disability
INTRODUCTION
Since the introduction of array comparative genomic hybridization (aCGH) into clinical practice, microduplications, and microdeletions (copy number variants, CNVs) have been shown to cause or contribute to a number of abnormal phenotypes, including autism, intellectual disability (ID), neurological disorders, epilepsy, and congenital anomalies [Cooper et al., 2011]. For this reason, aCGH has become a common diagnostic tool when searching for genetic causes of ID.
The Xp11.22-p11.23 region is rich in genes implicated in neurogenetic disorders and ID [Stocco dos Santos, et al., 2003; Hagens et al., 2006; Santos-Reboucas et al., 2011] and rich in rearrangement-prone sequence elements. However, breakpoints are inconsistent, making precise genotype-phenotype associations a challenge [Giorda et al., 2009]. A number of genes in the region have known effects due to point mutations, intragenic del/dups, or complete gene deletion: SHROOM4 (Stocco dos Santos X-linked mental retardation syndrome), KDM5C (non-syndromic ID), IQSEC2 (mental retardation, X-linked 1), and SMC1A (X-linked Cornelia de Lange Syndrome).
Duplications involving HUWE1 (HECT, UBA, and WWE Domains-Containing Protein 1) have been associated with non-syndromic ID [Madrigal et al., 2007; Froyen et al., 2012] (MIM 300705). However, not all duplications of Xp11.22-p11.23 in patients with ID include the HUWE1 gene, suggesting the involvement of other important ID genes in the Xp11.22-p11.23 region [Giorda, 2009; Yan et al., 2009; Holden et al., 2010]. We report a cohort of 13 patients with overlapping Xp11.22-p11.23 duplications to further study potential critical dosage-sensitive genes in the region (Table I).
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | F | F | F | F | F | M | M | F | M | M | M | M |
| Start (hg19) | 50112063 | 50112063 | 50112063 | 48120296 | 48322659 | 48420391 | 53198565 | 48310228 | 48310228 | 53558765 | 53548808 | 53548808 | 50350047 |
| Stop (hg19) | 50456458 | 50456458 | 50456458 | 52693966 | 52613026 | 50771856 | 53969809 | 52614912 | 52614912 | 53620255 | 54062110 | 54062110 | 54964018 |
| Size (Kb) | 344 | 344 | 344 | 4574 | 4290 | 2351 | 771 | 4305 | 4305 | 61 | 513 | 513 | 4614 |
| Inheritance | Mat | Mat | Unk | De novo | Unk | De novo | Mat | Unk | Unk | Unk | Mat | Mat | Mat |
| Other CGH Abnl: | Xp11.22 | Xp11.22 | Xp11.22 | N/A | 19p13.11 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Type | Dup | Dup | Dup | Dup | |||||||||
| Start | 53160114 | 53160114 | 53160114 | 19617878 | |||||||||
| Stop | 53713154 | 53713154 | 53713154 | 19897455 | |||||||||
| Size (Kb) | 553 | 553 | 553 | 280 | |||||||||
| Dup regiona | 1,2 | 1,2 | 1,2 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 1,2 |
| Relation | Proband | Mother of case 1 | Mat GM of case 1 | Proband | Proband | Proband | Proband | Proband | Proband | Proband | Brother of case 12 | Brother of case 11 | Proband |
| Demographics | |||||||||||||
| Age (y) | 17 | 40 | 66 | 6 | 7 | 11 | 5 | 6 | 14 | 1 | 3 | 7 | 1.5 |
| Weight (%ile) | 90 | 75 | 80 | >97 | 99 | 97 | 10-25 | 25-50 | 87 | 10 | 10 | N/A | <3 |
| HC (%ile) | 2 | 50 | 50 | N/A | 93 | 75 | 50 | 40 | 50 | 3 | 10 | 50 | 2 |
| Height (%ile) | 50 | >95 | 85 | N/A | 98 | 60 | 25–50 | 75 | 98 | 3–10 | 10–25 | 50 | 90 |
| Craniofacial | |||||||||||||
| Eye abnl | X | X | X | X | X | X | |||||||
| Synophrys | X | X | X | X | X | X | |||||||
| Ear abnl | X | X | X | X | X | X | |||||||
| Palate abnl | X | X | X | X | X | ||||||||
| Cardiovascular | |||||||||||||
| Dilated AR | X | X | |||||||||||
| Extremities | |||||||||||||
| Hand abnl | X | X | X | X | X | X | X | X | X | ||||
| Foot abnl | X | X | X | X | X | X | |||||||
| Gastrointestinal | |||||||||||||
| Constipation | X | X | X | X | X | ||||||||
| Neurological | |||||||||||||
| MRI abnl | X | X | |||||||||||
| ADD/ADHD | X | X | X | X | X | X | X | X | |||||
| Autism | X | X | X | X | |||||||||
| Dyslexia | X | X | X | X | |||||||||
| ID | X | X | X | X | X | X | X | X | X | X | X | ||
| Seizures | X | X | |||||||||||
| Speech delay | X | X | X | X | X | X | X | X | X | X | X | X | |
| Tantrums | X | X | X | X | X | ||||||||
| Motor | |||||||||||||
| Exercise intol | X | X | X | X | |||||||||
| FTT | X | X | X | X | X | X | X | ||||||
| Hypotonia | X | X | X | X | |||||||||
| Motor delay | X | X | X | X | X | X | X | X | X | ||||
| Endocrine | |||||||||||||
| Early menses (yrs) | N/A | X | X | X | N/A | X | N/A | N/A | N/A | N/A | N/A | N/A | |
| Other abnlb | X | X | X | ||||||||||
- Dup, duplication; mat, maternal; unk, unknown; CGH, chromosome genomic hybridization; GM, grandmother; y, years; %ile, percentile; HC, head circumference; Abnl, abnormality; AR, aortic root; ADD, attention deficit disorder; ID, intellectual disability; Intol, intolerance; FTT, failure to thrive; Adv, advanced; N/A, not applicable.
- a Region 1 is defined as 50,112,063-50,456,458 bp and Region 2 is defined as 53,160,114–53,713,154 bp.
- b Includes advanced bone age and other hormone abnormalities.
MATERIALS AND METHODS
The index patient was identified following clinical genetics referral. Additional patients with overlapping duplications were sought by querying Kaiser Permanente's Northern California cytogenetic database, supplemented with patients from Signature Genomics and through personal discussions with other researchers.
A clinical geneticist evaluated all patients. A phenotype questionnaire was created after careful review of previously published literature and our own phenotypic observation. Participation was completely voluntary and patients could choose to participate with or without providing photos. Written informed consent was obtained from all patients whose photographs were included in this publication. For the remainder of the patients, de-identified information was supplied by the patient's clinician.
Array CGH for Patients 1–5 was performed using the oligonucleotide-based, 60K, Sure Print G3 platform (Agilent Technologies, Santa Clara, CA). Patient 6 was analyzed using the bacterial artificial chromosome-based, whole-genome SignatureChip WG (Signature Genomics, Spokane, WA) [Ballif et al., 2008a], Patient 7 using the custom, oligonucleotide-based, 105 K SignatureChipOS v1 (Agilent Technologies, Santa Clara, CA) [Ballif et al., 2008b] and Patients 8–10 using the custom, oligonucleotide-based, 135 K SignatureChipOS v2 (Roche NimbleGen, Madison, WI) [Duker et al., 2010]. Array CGH for Patients 11–13 used the oligonucleotide-based, ExonArrayDx v.2.1 (GeneDx, Gaithersburg, MD) [Wong et al., 2008]. All aCGH results were originally reported in human genome build 18 (NCBI36/hg18) and subsequently converted to hg19 (GRCh37/hg19) for publication purposes using the UCSC genome conversion tool.
X-inactivation studies were performed in Patients 2 and 3 at Greenwood Genetic Center (Molecular Diagnostic Laboratory).
Control data were provided by the Wellcome Trust Case Control Consortium (WTCCC). Control CNV calls were derived from a subset of the control data sets published in Coe et al. [2014]. Specifically, Affymetrix SNP6 arrays from the WTCCC 2 58C, and newborn screening cohorts, as well as the Atherosclerosis Risk in Communities (ARIC) Community Surveillance Cohort (database of Genotypes and Phenotypes (dbGaP) accession phs000090.v1.p1) were processed using GTC4.1 as previously described [Coe et al., 2014]. After quality control, X-chromosome CNV calls were obtained for 6,459 males, and 7,236 females.
RESULTS
Patient 1: arr[hg19] Xp11.22(50,112,063–50,456,458)x2,Xp11.22(53,160,114–53,713,154)x2 mat
This male was born to a 22-year-old mother, who experienced severe preeclampsia requiring bed rest. He was born prematurely at 30 weeks gestational age, by emergency cesarean section for fetal distress. Birth weight was 1.9 kg (97th centile). He was hospitalized for 2 weeks due to feeding problems. He began to have staring spells starting at 7 months. At around 1 year of age, he was noted to have delays in motor and verbal skills and was soon diagnosed with cerebral palsy and probable seizures. At 5 years, he had surgery for strangulated testes. He walked at 2.5-years, talked at 2–3-years, and had simple reading skills at 10 years, which have regressed. Seizures started as absence seizures, but progressed to intractable grand mal seizures during high school. He was diagnosed with attention deficit hyperactivity disorder (ADHD) and had behavior problems and tantrums. Currently, he has severe ID and dysarthria with repetitive and obsessive speech. He follows only one step instructions and is easily distracted. He suffers from constipation and Crohn's disease. He has had declines in walking and coordination, and at the age of 18 years, had surgery for tethered cord, which resulted in improvements in gait, alertness, and a significant decrease in number of seizures. He has required testosterone therapy for hypogonadism and breast reduction surgery for gynecomastia, which began at age 14 years. He is also treated for hypothyroidism.
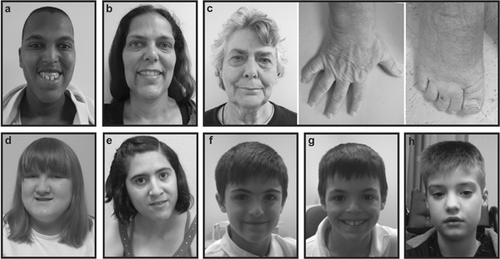
Physical exam at age 17 years was notable for long facies, high forehead, bilateral pterygia of the eyes, dysplastic ears with irregular outer helices, short philtrum, high and narrow palate, large mouth which is often open and drooling, and truncal obesity (Fig. 1a). He had tapered fingers, bilateral single palmar creases, left radio-ulnar synostosis, and mild contractures at the knees. Feet were flat with short toes, especially toes 4–5. Skin exam showed striae on arms, two cafe-au-lait spots, streaky hyperpigmentation on left breast, dry palms, and soles of feet. At the time of the exam, his weight was at the 75th centile, height was at the 50th centile, and head circumference was less than the 2nd centile.
MRI of the spine showed a lipoma of the filum terminale and six lumbarized vertebral bodies. There is acute kyphotic angulation at the sacro-coccygeal junction. Brain MRI showed several small patchy areas of high T2 signal in the periventricular and subcortical white matter, most compatible with small areas of gliosis/scarring, perhaps due to remote ischemic injury of prematurity. EEG showed bilateral frontal focal slowing. Other lab findings included: high TSH (7.4), low testosterone (206). Fragile X, peripheral blood karyotype and metabolic panels were normal.
There is a significant family history of multiple individuals with intellectual disability and mental illness. His mother (Patient 2) has mild intellectual disability; his maternal grandmother (Patient 3) has severe intellectual disabilities. The proband's only maternal aunt has a history of non-specific psychiatric problems and drug/alcohol abuse. This aunt has two sons and two daughters, all with mild to moderate learning disabilities. These individuals were not available for evaluation.
Patient 2 (Mother of Patient 1): arr[hg19] Xp11.22(50,112,063–50,456,458) x3,Xp11.22(53,160,114–53,713,154)x3 mat
This woman was born cyanotic to a 40-year-old mother after 27 hr of labor. Birth weight was 3.6 kg (50–75th centiles). She had problems nursing as an infant. She was in special education in high school and reported getting easily distracted, having lifelong constipation, bladder incontinence, early puberty (started 9-years-old), migraines, inflammatory bowel disease, asthma, and toe spurs. She has exercise intolerance, mild hearing loss, myopia, and dyslexia.
Physical exam at 40 years of age was notable for long facies, high forehead, prominent supraorbital ridges, midface hypoplasia, non-dysplastic ears, and tented upper lip (Fig. 1b). She wore braces for teeth crowding, had high-arched palate, and trismus. She had 5th finger clinodactyly, tapering fingers, deep-set first toenails, wide first toes, and high foot arches. Speech was normal. Her height was greater than the 95th centile and her head circumference was at the 50th centile.
Array CGH showed identical duplications to her son and mother, without additional pathogenic CNVs. X-inactivation studies showed moderately skewed X-inactivation pattern with 85% of the maternally inherited (abnormal) X-chromosome activated in the blood.
Patient 3 (Maternal Grandmother of Patient 1): arr[hg19] Xp11.22(50,112,063–50,456,458) x3,Xp11.22(53,160,114–53,713,154)x3
This female has a history of severe ID, speech delay, dysarthria, exercise intolerance, fatigue, constipation, and early puberty (8 years). As an adult, she developed diabetes, cataracts, myopia, dementia, and restless leg syndrome. She had a breast reduction in her 30's due to large breasts causing her back pain. She is described as having frequent tantrums and has a very similar personality to her grandson (Patient 1). She was being cared for by her daughter (Patient 2) and had never lived independently.
Physical exam at 65 years of age showed slight synophrys, dry palms and soles of her feet. She had tapering fingers with 5th finger clinodactyly, deep-set first toenails, wide first toes, bilateral 2–3 toe syndactyly, and hypoplastic fifth toenails (Fig. 1c). Her height was at the 85th centile, weight was at the 80th centile, and head circumference was at the 50th centile.
Array CGH identified the same two familial duplications found in her daughter and grandson without additional pathogenic CNVs. X-inactivation studies were uninformative.
Patient 4: arr[hg19] Xp11.23p11.22(48,120,296–52,693,966)x3 dn
At 6 years of age, this female patient was noted to have mildly dysmorphic features, mild ID, autistic spectrum disorder, and obesity. She had early puberty (9 years). She was mainstreamed in school and has been treated for attention deficit disorder. She had a square shaped nose, thin lips, small mouth, smooth long philtrum, cleft chin, small teeth with hypodontia, slightly low set ears with mild flattening of the superior helices, 5th finger brachydactyly, and mild clinodactyly (Fig. 1d). A bone age study, performed at nine years and 10 months, showed an advanced bone age of 13 years and 6 months.
Patient 5 arr[hg19] Xp11.23p11.22(48,322,659–52,613,026)x3,19p13.11(19,617,878–19,897,455)x3
This 6-year-old female had pseudostrabismus, hyperopia and astigmatism, dysmorphic features (mild hypotelorism, synophrys, small mouth, smooth philtrum, dentition with misalignment), mild to moderate ID, hoarse voice with articulation errors, delayed speech, social, fine motor and gross motor skills, behavioral problems, hirsute back, large slate-gray patch on lumbar area, and obesity.
Patient 6: arr[hg19] Xp11.23p11.22(48,420,391–50,771,856)x3 dn
This female was born at full term to a 25-year-old mother following an uncomplicated pregnancy. Hypotonia was noted at 3 months of age. Motor milestones throughout the first year were delayed. She sat up at about 1 year of age and walked at 2 years. Of note, she had pronation and in-toeing of her feet (requiring casting). Seizures were first documented by EEG at 7 years of age but, in retrospect, may have occurred much earlier. Advanced bone age was noted by 10 years. Throughout childhood, she needed special education with speech therapy. A developmental assessment at 11 years of age showed that overall she functioned cognitively at about a 5-year-old level and is described as having a shy and sensitive personality. A genetic physical exam showed subtle unusual features including prominent nasal bridge, synophrys (Fig. 1e), strawberry hemangioma on abdomen and tapered fingers with 5th finger clinodactyly. At 11 years old, her weight was at the 97th centile, height was at the 60th centile, and head circumference was at the 75th centile.
Routine karyotype, fragile X studies, FISH for 22q11.2 were normal. Array CGH with FISH confirmation (BAC clone RP11-1065H18) showed a pattern consistent with a duplication of Xp11.22. Parental FISH was normal.
Patient 7: arr[hg19] Xp11.22(53,198,565–53,969,809)x2 mat
This male has a history of speech and motor delay, mild ID, attention deficit hyperactivity disorder (ADHD), and history of failure to thrive. He had subtle dysmorphic features including a small face with a prominent forehead, and 5th finger clinodactyly. A Goldman–Fristoe Test of Articulation, administered at 6.5 years, showed speech articulation at the 2.5 year level. Fragile X studies and FISH for Cornelia de Lange syndrome were normal. His aCGH duplication was maternally inherited, confirmed by aCGH in his mother. No phenotypic information is available on his parents.
Patient 8: arr[hg19] Xp11.23p11.22(48,310,228–52,614,912)x2
This 6-year-old male has mild dysmorphic features and developmental delay. The patient also had a history of failure to thrive, which has resolved. Physical exam showed prominent eyebrows, synophrys, right sided cleft lip, cupped ears, elongated fingers, digitalized thumbs, language delay, dysarthria, attention deficit disorder, behavioral problems. MRI showed a small area of leukomalacia with left parietal white matter. Echocardiogram was normal.
Patient 9: arr[hg19] Xp11.23p11.22(48,310,228–52,614,912)x3
This 14-year-old female has speech delay with dysarthria, attention deficit disorder, autism, motor delay, dyslexia, and history of tantrums, and behavioral problems. Physical exam showed small eyes and tapering fingers. She also had a history of hypertension, constipation, and poor hygiene. Age at menstruation was 11 years. Normal labs included Fragile X, methylation studies for Prader Willi/Angelman syndromes, and metabolic panels. Interestingly, she has the same duplication breakpoints as in Patient 8, consistent with the previously described, recurrent, non-allelic homologous recombination (NAHR)-mediated 4.5Mb Xp11.22p11.23 duplication [Giorda et al., 2009]. There is no known familial relationship between these patients.
Patient 10: arr[hg19] Xp11.22(53,558,765–53,620,255)x2
This 1-year-old male has a history of global developmental delays including motor, verbal, and poor feeding coordination. He has reflux and dysphagia with the oral phase. At 1 year of age, he was only just beginning to sit on his own. Physical exam showed mildly dysmorphic features including bulbous nose, down-slanting palpebral fissures, simple ears but normally set, increased range of motion at small and large joints, and hypotonia. His height was at the 10th centile, weight was between 3rd and10th centile, and head circumference was at the 3rd centile.
Metabolic screens were essentially normal. Ophthalmology exam showed exotropia. Brain MRI was normal. Family history was limited due to social history. It is known, however, that he has a full brother with hypotonia and a maternal half-brother with autism. His mother, maternal grandmother, and maternal uncle were reported to have mild ID.
Patient 11 arr[hg19] Xp11.22(53,548,808–54,062,110)x2 mat
This male was noted to have speech delay at 19 months of age. During his second year, growth began to slow with height and weight crossing centiles. He was evaluated at 3 years of age due to failure to thrive and developmental delay. Physical exam showed mild synophrys, a broad nasal root, prominent metopic suture, relatively high palate, flat philtrum, and a midline gap with prominent front teeth that were serrated (Fig. 1f). Height was at the 10–25th centile, weight at the 10th centile and head circumference at the10th centile. Ear measurement was at the 3rd centile, and his palpebral fissures measured small. Cardiorespiratory and abdominal exam were normal. Musculoskeletal system showed bilateral clinodactyly with bilateral single palmar creases and abnormal dermatoglyphics. Neurological exam was normal. At 8 years of age, his height, weight, and head circumference were at the 10–25th centile. At 10 years of age, the patient was assessed as having probable dyslexia. At 12 years of age, he had aortic root dilation. Metabolic screening labs were normal. The Xp11.22 duplication was maternally inherited, confirmed by aCGH in his mother.
Patient 12: arr[hg19] Xp11.22(53,548,808–54,062,110)x2 mat
This male, brother of Patient 11, was also found to have to problems with speech development for which he has required speech therapy. In first grade, he had been assessed as probably dyslexic. He has a number of unusual facial features that are quite similar to his brother including: prominent forehead, broad nasal root, high palate, midline gap with prominent and serrated front teeth, and triangular shaped face (Fig. 1g). At 7 years of age, he was noted to have a bridged crease on his right palm and some mild clinodactyly on the second and fifth fingers. Head circumference and height were at the 50th centile. At 10 years of age, the patient was also found to have aortic root dilation.
Patient 13: arr[hg19] Xp11.22p11.21(50,350,047–54,964,018)x2 mat
Concerns about development were first raised at 4 months of age when this male patient had poor head control and hypotonia. Muscle strength, however, appeared to be relatively preserved. He was first evaluated in Genetics at 1 year of age. He had mildly dysmorphic features, long facies with bitemporal narrowing, and slightly protruding ears (Fig. 1h). At 16 months of age, he had generalized hypotonia, delay in his fine and gross motor skills, and delay in early language skills. Head circumference was at the 3rd centile, weight was below the 3rd centile, and length was at the 90th centile. At 2 years of age, he continued to have severe hypotonia, difficulty with many motor functions including reflux and vomiting, poor weight gain, and speech delay. At 7 years of age, he showed significant developmental delay and issues around anxiety and difficulty in adaptation. Head circumference remained just below the 2nd centile. MRI and EEG were normal. EMG showed a non-specific myopathic pattern. Muscle biopsy showed non-specific changes including some fibrosis and some atrophy.
Control Data
Control CNV calls, obtained for 6,459 males and 7,236 females, showed one small partial duplication, each, of DGKK (50,111,104–50,152,684 bp) and SHROOM4 (50,515,589–50,561,630 bp) in two male controls, a complete duplication of SMC1A in a female (53,408,909–53,488,545 bp), a partial duplication of HUWE1 (53,547,403–53,620,213 bp, and 53,556,873–53,628,051 bp) in two females. No larger duplication events were seen in the control sets.
DISCUSSION
Following detection of two microduplications in chromosome Xp11.22 in three syndromic family members (Patients 1–3), an effort was made to further delineate the critical ID genes in this region. Realizing that the proximal duplication contains the HUWE1 gene, which has been previously associated with isolated non-syndromic ID [Froyen et al., 2012], we aimed to characterize genotype-phenotype correlations in our cohort. We hypothesized that either of the following could explain the syndromic phenotype in the index family: (i) The duplication of the HUWE1 gene may be responsible for the ID phenotype in our Patients 1–3, with the syndromic features stemming from a different gene or set of genes. This hypothesis raised the possibility of a second candidate gene residing inside of the small distal duplication. (ii) The HUWE1 gene may be responsible for the ID and some or all of the syndromic phenotype, which would suggest variable expressivity and an expansion of the phenotype associated with HUWE1.
We describe the phenotype of 10 additional individuals with duplications overlapping either of the index family's duplications: six wholly or partially overlapping the distal “Region 1” (50,112,063–50,456,458 bp) containing only the SHROOM4 and DGKK genes and five wholly or partially overlapping the proximal “Region 2” (53,160,114–53,713,154 bp) containing 12 RefSeq genes including the HUWE1 gene (Fig. 2).

Region 1 (50,112,063–50,456,458 bp Within Chromosome Xp11.22)
Distal duplications at chromosome Xp11.23p11.22 in patients 1–6, 8, 9, and 13 spanned X-chromosome coordinates 48.1–55.0 Mb. The minimum region of overlap located at nucleotides 50,350,047–50,456,458 contains only the SHROOM4 (SHROOM Family Member 4, MIM 300579) locus.
In our dataset, all patients with duplications including Region 1 had mild-severe ID, 8/9 had a history of speech delay, 7/9 had attention deficit disorder, 7/9 had a history of motor delay, 6/9 had hand abnormalities (5th finger clinodactyly and/or tapering fingers), and 6/9 had foot abnormalities. Early onset of menses was common in females (4/5) with this duplication. Synophrys and constipation were each noted in 5/9 patients, and autism was seen in 4/9 patients. Interestingly, foot abnormalities, autism, and constipation were absent in patients who had only the Region 2 duplication (not also harboring the Region 1 duplication).
A missense mutation in SHROOM4 has been previously associated with X-linked intellectual disability in humans [Hagens et al., 2006]; however, the molecular mechanism is not understood. SHROOM4 has been showed to influence cytoskeletal architecture by modulating the spatial distribution of myosin II [Yoder and Hildebrand, 2007]. DGKK, duplicated in patients 1–6, 8, and 9, phosphorylates diacylglycerol, which serves to downregulate diacylglycerol signaling activity [Imai et al., 2005]. Two independent SNP association studies have shown strong associations with risk for hypospadias [van der Zanden et al., 2011; Carmichael et al., 2013]. Patient 1 has hypospadias.
Duplications including SHROOM4 and DGKK have been previously reported [Bonnet et al., 2006; Froyen et al., 2007; Marshall et al., 2008; Giorda et al., 2009]. Of particular interest is a report of a 15-year-old male with a duplication from 47.5–52.5 Mb and a remarkably similar phenotype to our patients [Bonnet et al., 2006]. He was described as having mental retardation, autistic-like behavior, speech delay, abnormal EEG, synophrys, early onset of puberty, abnormally folded helices of the ear. Additionally, Giorda et al., [2009] identified similar duplications (48.1–52.6 Mb) in males and females with borderline-severe ID, EEG abnormalities, speech delay, dysarthria, many of whom had hoarse/nasal voice, early puberty, and foot abnormalities. Marshall et al., [2008] identified a female with a de novo Xp11.22p11.23 duplication (48.0–52.7 Mb). This individual was described as having ID (IQ of 68), mild receptive and expressive language delay, moderate speech delay, autism, dysmorphic features, and severe hypotonia. Holden et al., [2010] reported a girl with de novo duplication at (40.4–52.7 Mb), described as having EEG abnormalities, brain MRI abnormalities, global developmental delay, expressive language delay, hypotonia, and indistinct speech.
Region 2 (53,160,114–53,713,154 bp Within Chromosome Xp11.22)
The proximal duplication in patients 1–3 spanned from 53.1 Mb to 53.7 Mb (Region 2) (Fig. 2). In addition, patients 7, 10–13 have duplications overlapping this region. This region contains seven MIM-annotated genes: KDM5C (Lysine-Specific Demethylase 5C; MIM 314690), IQSEC2 (IQ Motif- and SEC7 Domain-Containing Protein 2; MIM 300522), SMC1A (Structural Maintenance of Chromosomes 1A; MIM 300040), HSD17B10 (17-Beta-Hydroxysteroid Dehydrogenase X; MIM 300256), HUWE1 (HECT, UBA, and WWE Domains-Containing Protein 1gene; MIM 300697), MIR98 (Micro RNA 98; MIM 300810), and MIRLET7F2 (Micro RNA LET7F2; MIM 300721). It should be noted that the duplications in Patients 1–3 and 13 encompass both Region 1 and at least part of Region 2. In addition, Patient 10's duplication spans most, but not all, of the HUWE1 gene. In our dataset, among patients with duplications of this proximal region, 6/6 patients assessed had ID, 7/8 had speech delay, 6/8 had failure to thrive, 6/8 had hand abnormalities, and 5/8 had motor delay. Seizures were not a consistent finding in either the Region 1 or 2 groups.
Region 2 includes a number of genes which have been previously associated with ID. Loss-of-function mutations in KDM5C have been reported in patients with syndromic and non-syndromic mental retardation, autism spectrum disorders, and spastic paraplegia [Adegbola et al., 2008; Rujirabanjerd et al., 2010; Santos-Reboucas et al., 2011]. This gene is proposed to escape X-inactivation [He et al., 2011]. Missense or loss-of-function mutations in IQSEC2 have been associated with X-linked non-syndromic mental retardation with seizures, autistic traits, psychiatric problems, and delayed early language skills in males and females [Shoubridge et al., 2010; Tran Mau-Them et al., 2014]. Previous studies have suggested that IQSEC2 plays a role in regulation of actin cytoskeleton organization and neuronal development in the brain. This gene is also proposed to escape X-inactivation [He et al., 2011]. Dominant negative mutations in SMC1A are associated with X-linked Cornelia de Lange syndrome.
Froyen et al., [2008] previously reported a number of families with overlapping duplications including the HSD17B10 and HUWE1 Loci in the smallest region of overlap. The associated clinical phenotype was described as non-syndromic ID. Subsequent studies by the same authors, including mRNA studies and examination of breakpoints in additional families, suggest that the ID phenotype is due to overexpression of HUWE1 gene [Froyen et al., 2012]. Interestingly, patients with missense mutations in HUWE1 share clinical features with patients with a duplication of HUWE1. A similar phenomenon is observed in Pelizaeus–Merzbacher disease which is caused by a duplication, deletion, or missense mutation of the PLP1 gene [Woodward, 2008].
Our data suggest a number of possible conclusions regarding genotype-phenotype associations. First, Region 1, including the SHROOM4 and DGKK genes, may represent a new syndromic X-linked ID critical region, associated with a variable phenotype which can include precocious puberty, mild-severe ID, autism, speech delay, attention deficit disorder, constipation, motor delay, and foot abnormalities. Although the data suggest this association, we cannot exclude a contribution to the phenotype in some individuals from Region 2 or other genes in those patients with larger duplications. CNV control sets did not contain any individuals with complete duplications of SHROOM4 or DGKK. Additional studies will be needed to confirm this possible association.
Our data agree with previous studies, which have shown that sole duplications of Region 2, including the HUWE1 gene, are associated with mild-profound intellectual disability, speech delay, and failure to thrive. Although most previously reported patients appeared to be non-dysmorphic [Froyen et al., 2008], our data suggest that this region could be responsible for mildly dysmorphic features in some individuals including hand abnormalities and abnormal palate. Only one of our patients with the Region 2 duplication had seizures but this individual's duplication extended into Region 1. Interestingly, constipation, foot abnormalities, and exercise intolerance were not noted in any patients with Region 2 (not also harboring Region 1) duplications. Additional studies would be helpful in determining if Region 2 duplications are associated with a broader phenotype than previously described.
Proposed Mechanisms Promoting Duplication
Two studies have examined, in detail, the sequence elements within chromosome Xp11.23p11.22 that predispose this region to recombination and replication-based rearrangements [Giorda et al., 2009; Froyen et al., 2012]. In the case of the smaller HUWE1 duplications, the majority of the distal breakpoints map within two long-terminal repeats (LTRs) that flank or are located within the chromosomal domain previously shown to escape X-inactivation [Miller and Willard, 1998]. We hypothesize that the LTRs constitute chromatin boundary elements that demarcate a constitutively active chromatin domain that is more susceptible to endonuclease activity and, thus, more prone to genomic rearrangements than those regions undergoing chromatin compaction during X-inactivation (Fig. 3). Indeed, data from the DNA Encode project show an enrichment of DNAse1 hypersensitive sites within this region (UCSC Genome Browser).

In conclusion, our study adds to the growing number of cases of individuals with Xp11.22 duplications and adds unique information about possible genotype-phenotype associations in this region.
ACKNOWLEDGMENTS
We thank Dr. Julie Jones at Greenwood Genetic Center for X-inactivation studies.
This study makes use of data generated by the Wellcome Trust Case Control Consortium. A full list of the investigators who contributed to the generation of the data is available from http://www.wtccc.org.uk/.