Microform holoprosencephaly with bilateral congenital elbow dislocation; increasing the phenotypic spectrum of Steinfeld syndrome
Abstract
Steinfeld syndrome (MIM #184705) was first reported in 1982. It is characterised by holoprosencephaly and limb defects, however other anomalies may also be present. Following the initial description, three further cases have been reported in the literature. We report on a 23-year-old girl, with features of microform holoprosencephaly and bilateral congenital elbow dislocation in association with hypoplastic radial heads. She was identified to have a variant in the CDON gene inherited from her father who had ocular hypotelorism, but no other clinical features. We discuss the clinical features of Steinfeld syndrome, and broaden the phenotypic spectrum of this condition. Structural analysis suggests that this variant could lead to destabilisation of binding of CDON with hedgehog proteins. Further work needs to be done to confirm whether mutations in the CDON gene are the cause of Steinfeld syndrome. © 2016 Wiley Periodicals, Inc.
INTRODUCTION
Holoprosencephaly (HPE) is a common structural malformation resulting from failure of midline cleavage of the developing brain. HPE occurs in up to 1 in 250 conceptions although, the estimated prevalence in liveborn children is less than 1 in 10,000 [Orioli and Castilla, 2010]. HPE is typically classified into three types with increasing severity: lobar, semi-lobar, and alobar. However, milder forms are well documented and include middle interhemispheric variants, in which the posterior, frontal, and parietal lobes are incompletely separated and the corpus callosum may be hypoplastic [Simon et al., 2002], and microforms characterised by midline defects (e.g., single maxillary central incisor or hypotelorism) without the brain malformations typical of HPE [Nanni et al., 1999]. HPE is associated with environmental (e.g., teratogens) and genetic factors, including cytogenetic abnormalities and single gene defects coding various components of the Sonic hedgehog (SHH) signalling pathway [Roessler and Muenke, 2010]. Recently mutations in the cell adhesion associated, oncogene regulated gene (CDON), which encodes a hedgehog receptor, have been found to cause HPE [Bae et al., 2011].
In 1982, Steinfeld reported a female child with HPE, bilateral reduction defects of the upper limbs with dislocated shoulders. She also had cardiac and renal anomalies, and midline cleft lip, and palate. Other individuals within the family had variable limb reduction defects. Transmission appeared autosomal dominant. Since then, three further cases have been reported in the literature, all with HPE and limb defects among other features [Nöthen et al., 1993; Siebert et al., 2005; Stevens, 2010]. Until now, the genetic basis of Steinfeld syndrome has remained elusive. Investigation in previously reported cases has included standard karyotype, microarray-based comparative genomic hybridization (array CGH) and molecular testing of SHH, SIX3, TGIF, and ZIC2 genes.
Herein, we report a 23-year-old girl with features of microform HPE and bilateral congenital elbow dislocation in association with hypoplastic radial heads. She was identified to have a heterozygous missense variant in the CDON gene, inherited from her father who had minimal clinical features. It is possible that this is the cause of the features in this family. We discuss the clinical features of Steinfeld syndrome, and perform structural analysis to try to ascertain the nature of the CDON variant.
CLINICAL REPORT
This 23-year-old girl was referred for genetics assessment shortly after birth. She was the first child of healthy non-consanguineous Caucasian parents. There was no significant family history. She was born at 41 weeks gestation by normal delivery following an uncomplicated, teratogen and infection free pregnancy. Her birth weight was 2.87 kg (2nd–9th centiles). Shortly after birth, she was noted to have a flattened nose and hypoplastic midface. She subsequently developed breathing difficulties and underwent several procedures to straighten her nasal septum and dilate both the nostrils. A single central incisor was noted when her deciduous teeth erupted. She had one febrile convulsion at 3 years of age. Following eruption of her permanent dentition, orthopantomogram confirmed that she was missing four central incisors, the upper 5/5 (adult 2nd pre-molars) were missing with retained E/E (primary 2nd molars), and there was a possible supernumerary tooth in the upper left quadrant. At 18 years, she had a bony exostosis removed from her left humerus. She had mild-moderate learning difficulties.
On examination at 23 years of age, her height was 160 cm (25th centile), weight 58 kg (50th centile), and head circumference 50.5 cm (<0.4th centile). She had hypotelorism (palpebral fissure length 2.6 cm, intercanthal distance 2.3 cm) and a flattened hypoplastic nose with a deviated nasal septum (Fig. 1A). There was absent superior lip frenulum, a ridged palate with thickened alveolar margins, and a broad, grooved uvula. The apical impulse was present on the left side of the chest and there were no murmurs. She was unable to pronate or supinate both her elbows with no history of previous trauma.
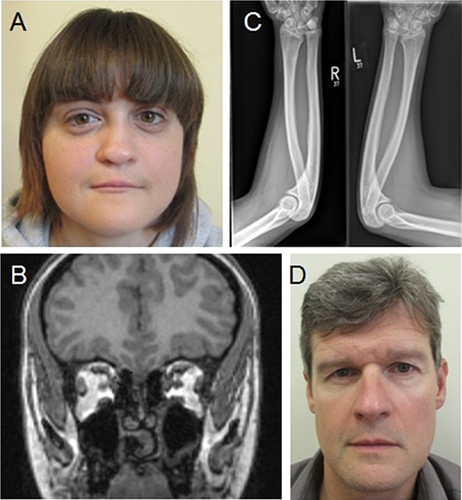
Ophthalmological examination at 1 year of age was unremarkable. Brain magnetic resonance imaging (MRI) revealed absent vomer (Fig. 1B); however there were no structural brain anomalies. Radiographs of her forearms identified longstanding bilateral elbow dislocation in association with hypoplastic radial heads (Fig. 1C). Echocardiogram and abdominal ultrasound scan were unremarkable. Array-CGH (Blue-Gnome Cytochip ISCA 8_60K Oligo Array; BlueGnome Ltd., Cambridge, UK) analysis identified a maternally inherited 0.25 Mb duplication within Xp22.32 (chrX:5441010-5697840 (hg19)). This region does not contain any annotated genes therefore it was thought unlikely to be clinically significant. Molecular testing by polymerase chain reaction (PCR) and next generation sequencing (NGS) of FGF8, GLI2, GLI3, PTCH1, SHH, SIX3, TGIF1, ZIC2, and MLPA analysis of GLI3, ZIC2, TGIF1, SIX3, SHH, GLI2, PTCH1 was negative. A heterozygous variant, c.2757G > C (p.Met919Ile) was identified in exon 15 of the CDON gene. The variant was paternally inherited, has not previously been reported and is located in a highly conserved nucleotide and amino acid position although results in small physiochemical difference.
We subsequently reviewed the father for features of holoprosencephaly. Clinical examination revealed ocular hypotelorism (palpebral fissure length 2.6 cm, intercanthal distance 2.4 cm), but no other abnormalities (Fig. 1D). Brain MRI, and radiographs of the forearms were unremarkable. Review of the family history revealed that the parents went on to have two further children. Their son was reported to have limitation of movement in his right thumb not related to previous trauma. He was not available for further assessment. There were no known abnormalities in the wider family.
METHODS
The CDON secondary structure elements were annotated with standard fibronectin type III domain structural features [Kang et al., 1997; Schultz et al., 1998; Finn et al., 2014; Letunic et al., 2015]. The structure of CDON mutant (M919I) was modelled using multiple templates from crystal structures pdb ID 3D1M, 3N1F, and 3N1Q [McLellan et al., 2008; Kavran et al., 2010] using the homology modelling software modeller version 9.11 [Eswar et al., 2007, 2008]. Of twenty model structures generated the model with lowest objective score was selected and used for structural analysis.
Alignment of model to crystal complexes and structural analysis was carried out using PyMOL (The PyMOL Molecular Graphics System, Version 1.3, Schrödinger, LLC, USA) as visualisation and locating residues in close proximity to the site of variant. For clarity when comparing the interaction of CDON and the three hhN domains, all hhN protein sequences are numbered according to the ShhN sequence numbering.
The residues in close proximity to the hedgehog protein binding partners were identified using PIC [Tina et al., 2007] LigPlot [Wallace et al., 1995] and EBI pdb analysis suite PDBsum [de Beer et al., 2014].
RESULTS
Structure and Role of CDO
The cell adhesion protein CDO (uniprot ID Q4KMG0) is a multidomain membrane spanning protein (Uniprot–Universal Protein Resource). The extracellular N terminal region comprises residues of 938 amino acids that form five Imunoglobulin-like (Ig) domains and three fibronectin type III (Fn III) domains, the transmembrane region comprises α helix of 21 amino acids in length with the cytoplasmic C terminal region consisting of 303 amino acids (Fig. 2A) [Kang et al., 1997]. There are three structures reported for CDO (PDB ID: 3D1M, 3N1F, and 3NIQ) all of which are restricted to the Fn III domain fragment from residue P826 to K924 [McLellan et al., 2008; Kavran et al., 2010]. In all three structures, the Fn III domain comprises eight β strands, seven of which arrange into two sheets comprising four and three strands; β1, β2, and β5 form one sheet with β3, β4, β6, and β7 form the other and together these sheets form a β-sandwich (Fig. 2A) [Schultz et al., 1998; Letunic et al., 2015]. The Fn III domain is also defined by a RGD sequence, a conserved motif that binds integrin yet is not present in CDO Fn III.
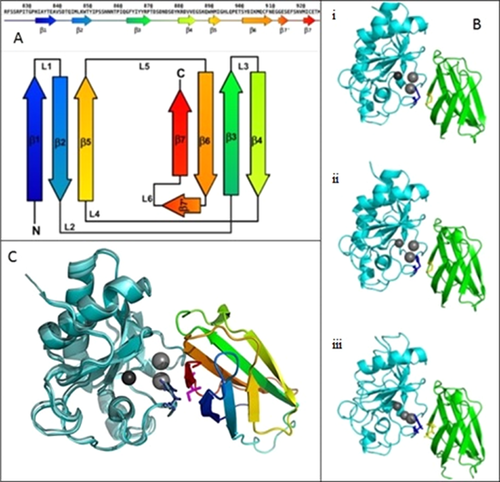
The role of CDO is regulatory and has been found to bind to a group of hedgehog domain (hh) proteins that are morphogens. There are three human hh proteins identified as regulated by CDO; indian hedgehog (Ihh), sonic hedgehog (Shh), and desert hedgehog (Dhh) which are involved in nervous system (Shh) [Ingham and McMahon, 2001], skeletal (Ihh and Shh) [Ingham and McMahon, 2001; Kronenberg, 2003], and testis development (Dhh) [Bitgood et al., 1996]. Regulatory control of these proteins is essential as abnormal hh signalling is associated with several cancers [Pasca di Magliano and Hebrok, 2003]. Critically mutations in human Sonic hedgehog protein have been identified previously as responsible for a significant proportion of autosomal dominant holoprosencephaly [Nanni et al., 1999; Roessler and Muenke, 2010].
Interaction of hh With CDO
The interaction of the N terminal domain of the hedgehog proteins (hhN) with CDO Fn III domain is conserved with its drosophila counterpart and is mediated by charged-charge interaction of residues D870 D872, E897, D901, E922 of CDO Fn III with R154, R124, K88, R156, and K179 of hhN [Wallace et al., 1995; de Beer et al., 2014]. These ionic interactions are critical as evinced by the pH dependent nature of the complex [Kavran et al., 2010]. In addition to the charged interaction, a hydrophobic component to the binding site is present between V918 M919 I920 of CDO and H134 of hhN.
The Met919Ile variant identified is located in the extracellular portion within the third Fn III domain that is located adjacent to the membrane spanning helix. The M919I forms part of the hydrophobic component of the binding site [McLellan et al., 2008] with M919I in particular close proximity with His 134 of ShhN domain (Fig. 2Bi). This proximity is also conserved in the homologous complex formed between CDO Fn III and IhhN or DhhN hedgehog proteins of drosophila (Fig. 2Bii and iii) [Kavran et al., 2010].
The Met919 is orientated towards the molecule surface as the methionine sidechain is exposed between the two sheets of the β-sandwich of the Fn III domain thus, it may be possible that the greater hydrophobic propensity of the Ile may result in this residue deriving a greater energetic penalty in this orientation. Furthermore, the branched nature of isoleucine results in a more bulky side chain that may induce distortion between the β sheets in order to alleviate steric clashes (Fig. 2C).
Although, the change from Methionine to Isoleucine would not necessarily change the potential for hydrophobic interaction, the specific free electron pair of the sulphur (δ) atom of methionine may specifically interact with the π electrons from the aromatic ring of histidine as indicated by the close proximity of the two in the crystal structures 3D1M, 3N1Q, and 3N1F at 4.28 Å, 4.48 Å, and 4.24 Å respectively between Methionine Sδ and Histidine Cδ2 atoms.
DISCUSSION
We report here the identification of a heterozygous variant in CDON in an individual with features of Steinfeld syndrome. This was inherited from her father who had ocular hypotelorism, but no other features of HPE. The proband's sibling had a possible radial ray defect, but was not available for clinical evaluation or genetic testing. Phenotypic variability and reduced penetrance have been well documented in HPE [Solomon et al., 2009]. Ming and Muenke [2002], suggested that HPE is a condition in which multiple genetic and environmental influences affect the severity of the phenotype and that multiple hits may be required for severe manifestations of the condition.
The original report by Steinfeld in 1982 described a severely affected female baby with HPE, midline cleft lip and palate, markedly shortened ulnas and radii, dislocated shoulder, absent thumbs, renal and cardiac anomalies, and an absent gallbladder. Five other individuals in the family were reported to have features. There was a perinatal death in a sister, and an aunt who died in infancy, both with similar features. The other affected individuals were reported to have absent thumbs and varying degrees of phocomelia. Nöthen et al. [1993], reported an 18 week fetus with alobar HPE, cyclopia, midline cleft lip and palate, absent nose, radial, cardiac, gallbladder, and vertebral anomalies. Siebert et al. [2005], reported a 20 week female fetus with semi-lobar HPE, micrognathia, cleft palate, severe phocomelia of all limbs, axial, cardiac, renal, and genitourinary anomalies. The most recently reported case was from Stevens [2010], who reported a 23 week old fetus with alobar HPE, abdominal situs inversus, severe limb defects, microphthalmia, bilateral cleft lip and palate, and cardiac anomalies. A further case of prenatally diagnosed HPE and radial defects has been reported but not attributed to Steinfeld syndrome [Dane et al., 2009]. To date all reported cases of Steinfeld syndrome have been associated with significant defects. This case broadens the spectrum of the condition to include milder forms with microform HPE and radial head hypoplasia associated with elbow dislocation, without limb shortening. Given the variable phenotype clearly documented with HPE, and other conditions with autosomal inheritance, it would not be surprising that Steinfeld syndrome is also a variable condition, with a degree of ascertainment bias in reporting towards the severe end of the spectrum.
At birth, the probands nasal hypoplasia was reminiscent of maxillonasal dysostosis as described by Binder [1962]. However, when the primary dentition erupted in our patient and a single maxillary central incisor was identified, a diagnosis of HPE was considered. The craniofacial malformations seen in HPE involve the median structures derived from the frontonasal process. This includes the interorbital region, nose, prolabium, ethmoid sinus, nasal bones, nasal septum, and premaxillary bones with the alveolar processes and the four maxillary incisors [Camera et al., 1992]. The additional features in our case of microcephaly, absent superior lip frenulum and developmental delay, are in keeping with this diagnosis [Martin and Jones, 1998; Heussler et al., 2002; Solomon et al., 2012]. Stevens [2010] provided an excellent review with differential diagnoses of HPE, and limb reduction defects. Other diagnoses considered included DK-phocomelia, Hartsfield syndrome, XK-aprosencephaly, and pseudotrisomy 13, although, there was no encephalocele, ectrodactly and hypertelorism, structural brain anomalies, or polydactyly respectively to suggest these diagnoses. We feel that the features in our case would be most consistent with a mild form of Steinfeld syndrome.
Following the reports that Cdon−/− mice exhibit features of holoprosencephaly with variability of phenotype [Cole and Krauss, 2003; Zhang et al., 2006], Bae et al. [2011] identified different heterozygous CDON mutations in six unrelated cases of human HPE. The CDON mutations identified resulted in loss of ability to promote SHH signalling. The six reported cases exhibited a severe phenotype. No limb defects were documented. In the three cases, where inheritance was ascertained, two were inherited, the remaining case was de novo. In the report, there were no clinical details for the affected parents. Since this initial report, there have been no other reported cases of HPE associated with CDON mutations, suggesting that they are a rare cause of genetic HPE.
The CDON variant p.Met919Ile identified in our case has not been reported previously. Structural analysis concluded that the branched nature of the Isoleucine creates a greater potential for steric clash in the M919I mutant, which distorts the beta sheet binding surface that interacts with residues of the hhN domains in the modelled structure. This could potentially disrupt the coordination of the neighbouring calcium ion (Fig. 2C). The lone electron pairs on the sulphur also come into contact with the histidine 134 hhN domains in the crystal structure, the Isoleucine is unable to form these contacts and thus, may destabilise the histidine orientation at the protein-protein interface which again can potentially destabilise the binding of the hhN domain. This destabilisation of CDO:SHH complex could lead to disruption of the SHH signalling pathway, and therefore, features of HPE.
The Hedgehog family of signalling molecules form a key regulatory network, conserved from vertebrates to invertebrates, controlling multiple developmental processes [Sasaki et al., 1999], including craniofacial, central nervous system, and limb development. Although, limb anomalies are not a typical feature of HPE, alterations in genes within the hedgehog pathway have been associated with limb abnormalities. In particular, postaxial polydactyly has been associated with mutations in GLI2 [Bertolacini et al., 2012]. It has been suggested that GLI3 and PTCH2 may be important for limb development [Akiyama et al., 2015; Zhulyn et al., 2015]. However, clearly the majority of cases of HPE associated with mutations within the hedgehog pathway are not associated with limb defects, therefore, it may be that other genetic and environmental factors play a role in the development of the limb anomalies of Steinfeld syndrome.
In summary, we report a 23-year-old girl with features of Steinfeld syndrome expanding the clinical phenotype for this rare condition. We identified a heterozygous missense variant in the CDON gene in the proband and her father who had ocular hypotelorism only. Structural analysis has suggested that this variant alters the structure of the protein such to disrupt the binding with other components of the hedgehog pathway. Additional clinical reports of Steinfeld syndrome including analysis of the CDON gene would be required to confirm the association and to further delineate this condition.
ACKNOWLEDGMENT
The authors would like to thank the family for agreeing to the publication of clinical details.




