Sprengel anomaly in deletion 22q11.2 (DiGeorge/Velo–Cardio–Facial) syndrome
Abstract
Sprengel anomaly (SA) is a rare skeletal defect characterized by uni- or bi-lateral elevation of the scapula. This anomaly is often isolated, although it can occur in association with other defects, including cervical spine malformations, cleft palate, and facial anomalies. Neural crest migration anomalies have been involved in the etiology of SA. Since the same embryological pathway accounts for some of the clinical features of deletion 22q11.2 syndrome (del22q11.2; DiGeorge/Velo–Cardio–Facial syndrome), we investigated the occurrence of SA in a consecutive series of 235 del22q11.2 patients aged more than 2 years, undergoing a complete clinical and orthopedic assessment of the dorsal and thoracic skeleton. In the present series, two patients were diagnosed with true SA. Present results and published reports suggest that scapular involvement including SA occurs in 1–2% of del22q11.2 individuals. Accordingly, this anomaly should be investigated as one of the possible skeletal findings of del22q11.2 syndrome, while this diagnosis should be excluded in patients presenting with SA associated with other defects. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Sprengel anomaly (SA) is a rare skeletal defect characterized by uni- or bilateral elevation of the scapula [Williams, 2003]. This anomaly is often isolated, but it can occur in association with other defects, including cervical spine malformations, cleft palate, and facial anomalies [Harvey et al., 2012].
Only limited data are available on the embryological origin of scapula, but a link between the development of malformations in the neck and shoulder regions has been suggested. The evidence of SA in several disorders in which a vascular etiology has been proposed, including Poland syndrome [Yiyit et al., 2015], could suggest the same etiology for the SA [Bavinck and Weaver, 1986]. However, the skeleton affected in SA is considered a neural crest derivative. The same pathway has been implicated in other malformation disorders affecting the neck and shoulder region, including Klippel–Feil anomaly and Arnold–Chiari I/II malformation [Matsuoka et al., 2005].
Since neural crest migration anomalies have been implicated in some defects associated with deletion 22q11.2 syndrome (del22q11.2) or DiGeorge/Velocardiofacial syndrome (DGS/VCFS), we investigated the occurrence of SA in a large series of patients with this disorder.
MATERIALS AND METHODS
A retrospective series of 307 patients with del22q11.2 consecutively investigated at the Bambino Gesù Pediatric Hospital and the Sapienza University of Rome in years 2002–2014 was enrolled in this study. Criteria for inclusion were an age of more than 2 years (range 2–27 years), clinical orthopedic assessment, and radiographic examination of the vertebral column, scapulae and thoracic cage. In all cases the diagnosis was confirmed by Fluorescent in Situ Hybridization (FISH), using a N25-D22S75 DGCR probe, or by Multiple Ligation Probe Amplification (MLPA), using P023-B2 and P250-B1 kits and capillary electrophoresis on an ABI-Prism 3130 Genetic Analyzer.
RESULTS
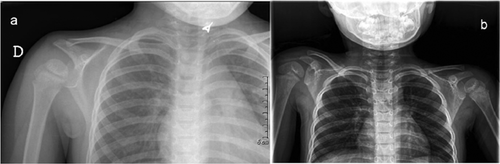
A total of 235 patients were available for the study. All patients had clinical examination, 187 had clinical orthopedic evaluation, and 112 radiographs of the vertebral column,scapulae and thoracic cage. A diagnosis of SA was reached in 2/235 (0.8%) subjects. Both patients showed a grade 2–3 SA following the Cavendish Classification [Cavendish, 1972]. No surgical correction was needed. The two patients with SA are described in the Clinical Reports, and summarized in Table I. Moreover the radiographic findings are shown in Figure 1.
| Clinical features | Patient 1 | Patient 2 |
|---|---|---|
| Age (years) | 4.9 | 2.6 |
| Sex | Female | Female |
| Weight (centile) | 75th | <3rd |
| Stature (centile) | 3rd–10th | 10–25th |
| Head circumference (centile) | 10th | 10–25th |
| Facial anomalies | + | + |
| Periorbital fullness | + | + |
| Upslanting palpebral fissures | + | + |
| Tubular nose | + | + |
| Everted upper lip | + | + |
| Dysmorphic ears | − | + |
| Palatal anomalies | + | + |
| Bifid uvula | + | − |
| Velo-pharyngeal insufficiency | + | + |
| Submucous cleft palate | − | + |
| Dental enamel hypoplasia | − | + |
| Congenital heart defect | − | + |
| Ventricular septal defect (muscular) | − | + |
| Renal anomaly | − | − |
| Cervical malformation | − | − |
| Conductive deafness | + | + |
| Slender fingers | − | + |
| Delayed motor milestones | − | + |
| Speech delay | + | + |
| Cognitive deficit | − | + |
CLINICAL REPORTS
Patient 1
The patient, a female, was the first child of healthy non-consanguineous parents. At delivery, the mother was 30 years old, and the father 31. The proband's sister was healthy. The patient was born at term of an uneventful pregnancy, with a birth weight of 3050 g (50th centile), length 50 cm (50th centile), and head circumference 34 cm (25th centile). Apgar score was 9 and 10 at 1 and 5 min.
The patient was first evaluated by us at 4 years and 9 months of age. Weight was 17 kg (75th centile), height 98 cm (3rd–10th centile), head circumference 49 cm (10th centile). Clinical examination revealed periorbital fullness, upslanting palpebral fissures, tubular nose, everted upper lip, bifid uvula, and SA of the right shoulder. Motor developmental milestones were normal, minor speech difficulties were noted, and the voice was hypernasal. Mild velopharyngeal insufficiency was documented by nasopharyngoscopy. Pure-tone audiometry showed mild conductive deafness, and history of frequent otitis media was documented. Skeletal radiographs showed upward shifting of the right scapula without malformations of the vertebral column (Fig. 1a). In addition, the dens of the second cervical vertebra (or epistropheus) was grossly triangular. Doppler two-dimensional echocardiography and renal ultrasonography were unremarkable. Magnetic resonance imaging of the heart and of the aortic vessels showed no abnormalities. Standard chromosome analysis was normal, while a de novo 22q11.2 deletion was detected by FISH using the N25 probe, and confirmed by MLPA which disclosed the common 3 Mb deletion inside the 22q11.2 critical region.
Patient 2
The patient, a female, was the second child of healthy non-consanguineous parents. At delivery, both parents were 38 years old. The proband's sister and brother were healthy. The patient was born at term after an uneventful pregnancy, with a birth weight of 2900 g (25–50th centile), length 49 cm (25–50th centile), and head circumference 33 cm (10–25th centile). Apgar scores were 9 and 10 at 1 and 5 min.
The patient was first evaluated by us at 2 years and 6 months of age. Weight was 10.2 kg (<3th centile), height 86 cm (10–25th centile), head circumference 47 cm (10–25th centile). Clinical examination showed periorbital fullness, upslanting palpebral fissures, tubular nose, mouth with downturned corners, narrow palate, submucous cleft palate, dental enamel hypoplasia, small dysmorphic ears, SA of the right shoulder, slender fingers and hypotonia. Developmental milestones and language were mildly delayed. The voice was hypernasal, due to velopharyngeal insufficiency. At birth, 2-Dimensional color-Doppler echocardiography showed muscular ventricular septal defect and patent foramen ovale. Spontaneous closure of cardiac defects was documented by echocardiography at age 1 year. Skeletal radiographs showed asymmetric shoulders with an elevation of the right scapula. Vertebral column was mildly left deviated, but no vertebral malformation was detected (Fig. 1b). Pure-tone audiometry showed mild conductive deafness. Renal ultrasonography was unremarkable. Standard chromosome analysis was normal, while a de novo 22q11.2 deletion spanning 3 Mb of the DGS/VCFS critical region was diagnosed by FISH and MLPA.
DISCUSSION
Sprengel anomaly is the most common malformation of the scapula. It can be isolated or associated with other defects, such as scoliosis, hemivertebrae, segmentation anomalies of vertebrae and ribs (including Klippel–Feil sequence), spina bifida, neck and shoulder muscles hypoplasia, and clavicular anomalies.
The genetic etiology of SA is supported by possible association with different disorders, including Klippel–Feil [Hensinger et al., 1974], Poland [Yiyit et al., 2015], Greig [Keats, 1970], Gorlin [Snoeckx et al., 2008], Floating–Harbor [Hersh et al., 1998], Goldenhar syndromes [Avon and Shively, 1988], and VACTERL Association [Fernback and Glass, 1988]. In addition, familial inheritance of isolated SA has been reported in a few pedigrees [Gottesleben, 1927; Kaissi et al., 2005; Wilson et al., 1971].
Little is known about the embryological origin of the scapula, and its relationship with associated defects. It is likely that the origin and genetic control of scapula are similar to those of the spine. This could explain the high concurrence of vertebral and scapular anomalies. A milestone study identified SA as a neural crest derived skeletal structure, mapping a cryptic neural crest mesoderm boundary inside the neck and shoulder girdle skeleton in which cellular distributions of neural crest and mesoderm correspond precisely to muscle attachment scaffolds to the shoulder girdle [Matsuoka et al., 2005]. Neural crest cells anomalies have been related to some clinical features of del22q11.2 syndrome [Walker and Trainor, 2006]. This supports an association between SA and this disorder.
Skeletal anomalies are found in 10–25% of the DGS/VCFS patients, including scoliosis, vertebral malformations (butterfly vertebrae, hemivertebrae, vertebral fusion of the cervical spine, and coronal clefts), talipes equinovarus, radial defects, polydactyly (pre- or post-axial of the hands, post-axial of the feet), and camptodactyly [Cormier-Daire et al., 1995; Digilio et al., 1997; Ming et al., 1997; Ryan et al., 1997; Matsuoka et al., 1998; Sabry et al., 1998; McDonald-McGinn et al., 1999; Vantrappen et al., 1999; Adachi et al., 2003].
Scapular involvement including SA has been reported as an occasional feature of del22q11.2 syndrome. In fact, an hypoplastic scapula is mentioned in 1.5% in a cohort of 250 cases [McDonald-McGinn et al., 1999], while SA and/or other scapular deformation were listed as one of the 185 clinical features of VCFS [Shprintzen, 2005]. SA was found in 1/38 patients included in the series by Lipson et al. [1991]. A skeletal radiographic evaluation is needed to discern true SA and other malformations or disorders that mimic SA. Previous reports do not specify the severity of SA or the need of surgical correction in del22q11.2 syndrome. In regard to the patients included in the present study, they showed a similar shoulder involvement of grade 2–3 following the Cavendish Classification, which did not require a surgical approach.
The association of SA, cleft palate, and cervical anomalies is quoted as Hodgson–Chiu syndrome (McKusick 184400), based on the original report of 1981 referring to a family in which SA and cleft palate segregated in an autosomal dominant fashion [Hodgson and Chiu, 1981]. Cervical spine anomalies were recorded in one affected patient. The existence of this syndrome was corroborated by the description of an additional familial case [Monier et al., 2000]. The association of SA with bifid uvula in one patient and submucous cleft palate in another subject from this report and the occurrence of cervical anomalies in del22q11.2 syndrome suggest that SA and Hodgson–Chiu syndrome may be part of the phenotypic spectrum of del22q11.2.
In conclusion, present findings and published results indicate that scapular involvement including SA occurs in about 1-2% of del22q11.2 patients. Accordingly, this anomaly should be investigated as part of the possible skeletal findings of del22q11.2 syndrome. Similarly, the diagnosis of del22q11.2 syndrome should be considered in patients with SA presenting with associated anomalies.




