A new case of bent bone dysplasia—FGFR2 type and review of the literature
TO THE EDITOR
Bent bone dysplasia syndrome (BBDS, MIM614592), was recently described by Merrill et al. [2012]. This skeletal dysplasia is characterized by bent long bones, diffuse osseous hypomineralization, hypoplastic pubis and clavicles, craniosynostosis and facial dysmorphism. It is usually lethal perinatally. BBDS is caused by heterozygous mutation in the FGFR2 gene (fibroblast growth factor receptor 2, MIM176943) on chromosome 10q26. Only five cases have been reported so far [Merrill et al., 2012; Scott et al., 2014]. Here, we report on a new case of BBDS and review the clinical and molecular findings of the previous cases.
The patient, a girl, was the fourth child of a non consanguineous couple of Moroccan origin. The parents and the first three children were healthy. There was no particular familial history. At conception, the mother was aged 41, and the father was 42 years old. First trimester of the pregnancy was unremarkable. Abnormalities were identified by ultrasound scan during the second trimester: cystic hygroma, short, and bowed long bones (femur, radius, and humerus), hypoplastic nasal bones, and hydramnios (Fig. 1). The fetal mobility was normal. Amniotic fluid drainage was performed at 28 WG. Karyotyping was normal 46,XX in standard resolution. Viral screening was negative. A second amniotic fluid drainage was necessary at 31WG because of polyhydramnios. The third trimester scan showed a protruding tongue (Fig. 1). The labor occurred spontaneously at 32 weeks of gestation and 5 days. The Apgar score was 4/4/1. Birth weight, length, and head circumference were 2770 g (75th centile), 45.5 cm (50th centile), and 33 cm (50th centile). Extra-uterine adaptation was inadequate necessitating an immediate resuscitation. She presented dysmorphic facial features with very large anterior fontanelle, down-slanting palpebral fissures, proptosis, severe hypoplasia of the middle face with bilateral choanal atresia, short nose with anteverted nares, macroglossia with protrusion of the tongue, and ear dysplasia. There was a rhizomelic shortening of the four limbs and an enlarged liver. Cardiac ultrasounds identified an atrial septal defect, ophthalmological examination showed an optic nerve atrophy, and auditive evoked potentials were absent. Skeletal X-rays showed hypoplastic clavicles, shortening of the long bones, bowed femurs, and a diffuse diminished mineralization particularly at the calvarium (Fig. 2). There was no significant platysplondyly on the front view, but side view of the spine is not available. Cerebral tomodensitometry and MRI were normal. Metabolic screening was normal. She died at 23 days of life because of respiratory failure. No autopsy was performed. Sequencing of FRFR2 identified the heterozygous mutation c.1141T > G (p.Tyr381Asp) in exon 11. Parental analyses were declined. However, the pedigree, the advanced paternal age at conception and the previous report of this mutation in BBDS were consistent with a de novo pathogenic event.
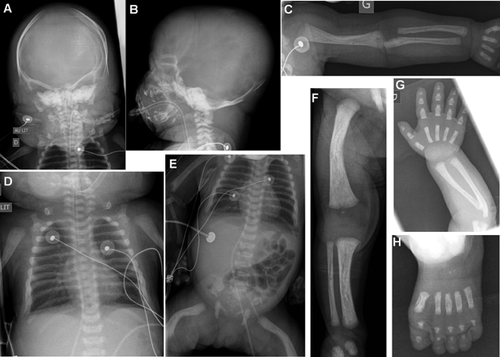
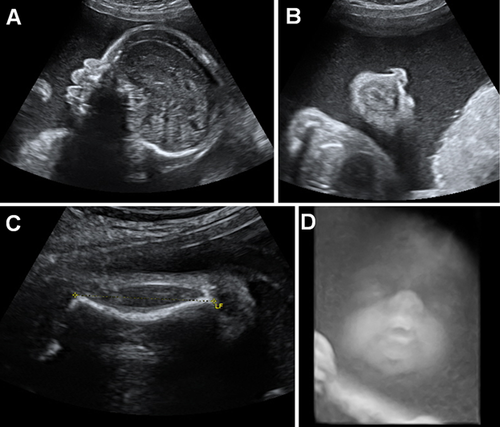
This case is the sixth reported case with BBDS FGFR2-type, and the second postnatal case [Merrill et al., 2012; Scott et al., 2014]. Reviewing of the clinical features of the reported cases shows that BBDS is a recognizable condition associating bent long bones (affecting more the lower than the upper limbs), craniosynostosis, exorbitism, and mid-face hypoplasia (Table I). Skeletal features are typical, showing deficient skull ossification, hypoplastic clavicles, pubis and ischia, brachyphalangy with angel-shapes phalanges (Table I). No fetal 3-D CT scan has been performed in the cases reported. In antenatal, this examination would probably help to classify the type of osteochondrodysplasia, as it is more efficient than ultrasound scans to describe precisely the skeletal anomalies. Histologic analysis of the distal femoral growth plate from two fetuses showed smaller hypertrophic chondrocytes, thickened, and hypercellular periosteum [Merrill et al., 2012].
| Merrill et al. [2012] R08–041 | Merrill et al. [2012] R07–401 | Merrill et al. [2012] R96–252 | Merrill et al. [2012] R05–427 | ||||
|---|---|---|---|---|---|---|---|
| Scott et al. [2014] | This report | Total | |||||
| Antenatal | ND | ND | ND | ND | Short long bones | Short and bowed long bones, cystic hygroma, tongue protrusion, hydramnios | |
| Birth term | ND | 23 WG | ND | ND | 32 WG | 32 WG | |
| Age at last examination | ND | Foetus 23 WG | ND | ND | Alive at 16 months | Died at 23 days | |
| Dysmorphism | |||||||
| Large anterior fontanelle | ND | ND | ND | ND | + | + | 2/2 |
| Hypertelorism | + | + | ND | + | + | + | 5/5 |
| Prominent eyes | + | + | ND | + | + | + | 5/5 |
| Mid-face hypoplasia | + | + | ND | ND | + | + | 4/4 |
| Micrognathia | + | + | ND | ND | + | + | 4/4 |
| Skeletal features | |||||||
| Choanal atresia | ND | ND | ND | ND | − | + | 1/2 |
| Craniosynostosis | + | + | − | + | + | − | 4/6 |
| Deficient skull ossification | + | + | + | + | + | 6/6 | |
| Hypoplastic clavicles | + | + | + | + | + | + | 6/6 |
| Hypoplastic pubis and ischia | + | + | + | + | + | + | 6/6 |
| Bowed femur | + | + | + | + | + | + | 6/6 |
| Bowed tibia/fibula | + | + | + | + | + | + | 6/6 |
| Brachyphalangy | + | + | + | + | + | + | 6/6 |
| “Angel-shaped” phalanges | + | ND | ND | ND | + | + | 3/3 |
| Other | |||||||
| Respiratory compromise | ND | ND | ND | ND | + | + | 2/2 |
| Hepatomegaly | + | − | − | − | − | + | 2/6 |
| Abnormal genitalia | Clitoridomegaly | Clitoridomegaly | ND | Clitoridomegaly | Hypospadias, undescended testes | − | 4/5 |
| FGFR2 mutation | p.Met391Arg | p.Met391Arg | p.Met391Arg | p.Tyr381Asp | p.Tyr381Asp | p.Tyr381Asp | 6/6 |
- NA, not applicable; ND, not determined.
FGFR2 gene encodes a transmembrane tyrosine kinase receptor which is an important regulator of bone formation during embryonic development. The protein has an extracellular ligand-binding region (immunoglobulin-like domains IgI, IgII, and IgIII), a transmembrane region (TM), and a tyrosine kinase domain (TK1 and TK2). FGFR2-related disorders comprise syndromic or non-syndromic craniosynostosis. Among the syndromic conditions (Apert, Crouzon, Pfeiffer, Beare-Stevenson Cutis Gyrata, and Jackson–Weiss syndromes), congenital hands and feet malformations are frequently associated, but bowed and short limbs are not typically described [Johnson and Wilkie, 2011]. In these disorders, genotype-phenotype correlations are observed: two missense mutations located in the linker between IgII and IgIII domains are responsible for Apert syndrome (p.Ser252Trp and p.Pro253Arg), while most mutations responsible for Pfeiffer or Crouzon syndromes are located in the IgIII domain (exons IIIa or IIIc) [Johnson and Wilkie, 2011]. Moreover, Beare–Stevenson Cutis Gyrata syndrome is caused by two specific mutations: p.Ser372Cys located in the linker region between IgIII and TM, and p.Tyr375Cys mutation located in the TM domain [Przylepa et al., 1996].
Our case shares the same mutation c.1141T > G (pTyr381Asp) with previously reported BBDS cases, strengthening its pathogenicity [Merrill et al., 2012; Scott et al., 2014]. The mutation lies in the transmembrane domain and induces the substitution of a highly conserved hydrophobic residue by a negatively charged polar amino acid in the TM helix [Merrill et al., 2012]. Previous functional studies found that this mutation reduces canonical signaling and has an enhancing effect on nucleolar occupancy of FGFR2 at the ribosomal DNA promoter, therefore increasing osteoprogenitors proliferation and decreasing cell differentiation [Merrill et al., 2012; Neben et al., 2014]. The Tyr-381 residue is present in human FGRF1-3 and in the two major FGFR2 isoforms [Collet et al., 2014]. A mutation at the same residue (p.Tyr381Asn, c.1141T>A) was identified in a familial case of Crouzon syndrome [Collet et al., 2014]. There were three cases, one with a typical Crouzon syndrome and two related individuals with milder phenotype consisting of fusion of the sagittal suture. The Asparagine substitution had no impact on the FGFR2 transmembrane topology [Collet et al., 2014]. This finding is likely to explain the milder phenotype observed, compared to BBDS.
Several differential diagnoses for BBDS have already been discussed elsewhere [Scott et al., 2014]: other FGFR2-related disorders, campomelic dysplasia (SOX9 gene), Antley–Bixler syndrome (POR gene), otopalatodigital disorders (FLNA gene), osteoglophonic dysplasia (FGFR1 gene), Cole–Carpenter syndrome (unknown gene), and thanatophoric dysplasia type 1 (FGFR3 gene). In addition, other conditions seem relevant differential diagnoses. Among the diagnoses comprising hypomineralization, some have additional features overlapping with BBDS: bent bones can be found in fetal Hypophosphatasia while hypoplasia of the clavicles, pubis, and ischia are observed in Cleidocranial dysostosis. However, the characteristic facial dysmorphism of BBDS can easily help to rule out these diagnoses. Furthermore, platyspondyly is observed in fetal cases of Hypophosphatasia but seem to be absent in BBDS. In our case, the first hypothesis regarding the craniofacial dysmorphism and the skeletal dysplasia was Raine syndrome (MIM 259775). This lethal osteosclerosis bone dysplasia is a rare autosomal recessive disorder due to mutations in FAM20C gene on 7p22.3 [Faundes et al., 2014]. It is characterized by a generalized osteosclerosis with a periostic ossification, and bowing of the long bones can be observed. The craniofacial dysmorphism associates a large anterior fontanel, an exorbitism, a short nose with depressed nasal bridge, a mid-face hypoplasia responsible for choanal stenosis, or atresia. These facial features are strikingly similar to BBDS. X-rays usually show generalized osteosclerosis but sometimes focal with a periostic bone formation. The osteosclerosis and the absence of bent bones may help to differentiate Raine syndrome from BBDS where the mineralization is diminished (especially marked for the skull). Furthermore, intracranial calcifications and elevation of serum alkaline phosphatase are frequently observed in Raine syndrome, these findings being absent in BBDS. Based on this first diagnosis hypothesis, sequencing of FAM20C was performed in our case, revealing no mutation in the coding regions.
BBDS is a rare and recently described skeletal dysplasia, still under-recognized by the radiologists, and geneticists specialized in constitutional bone diseases. Reviewing of the clinical and skeletal features may help the diagnosis, which can be easily confirmed by sequencing of FGFR2. This is of importance for the genetic counseling as this condition occurs de novo, while some differential diagnoses may be associated with a recurrence risk.




