Isolated bilateral transverse agenesis of the distal segments of the lower limbs at the level of the knee joint in a human fetus
Abstract
Congenital limb anomalies occur in Europe with a prevalence of 3.81/1,000 births and can have a major impact on patients and their families. The present study concerned a female fetus aborted at 23 weeks of gestation because she was affected by non-syndromic bilateral absence of the zeugopod (leg) and autopod (foot). Autopsy of the aborted fetus, X-ray imaging, MRI, and histochemical analysis showed that the distal extremity of both femurs was continued by a cartilage-like mass, without joint cavitation. Karyotype was normal. Moreover, no damaging variant was detected by exome sequencing. The limb characteristics of the fetus, which to our knowledge have not yet been reported in humans, suggest a developmental arrest similar to anomalies described in chicks following surgical experiments on the apical ectodermal ridge of the lower limbs. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Limb anomalies have a prevalence of 3.81/1,000 births in Europe if one excludes chromosomal disorders, according to EUROCAT registers [Dolk et al., 2010]. They involve multiple phenotypes, with heterogeneous etiologies. Their mechanisms are poorly understood, though a few genetic pathways have been defined. Indeed, transgenic mice misexpressing genes involved in limb patterning have been observed to have missing phalanges [Hummel, 1970], tibia [Chen et al., 2004], or femur [Williams et al., 2006], but no tibio-fibular shortening. In humans, similar phenotypes do not necessarily correspond to the same misexpressions and systematically involve nonskeletal organs [Goodman et al., 2000]. The present study concerns a human fetus with bilateral terminal transverse lower limb defect, without any associated disorder. To the best of our knowledge, this anomaly has not been described in the literature. Its description questions the understanding of lower limb development.
CLINICAL REPORT
The female fetus was the third child of healthy non-consanguineous Caucasian mother and father, who were 36 and 32 years old, respectively, at the time of conception. Except for one paternal uncle of the father suffering from trisomy 21, there was no known family history of genetic anomaly.
As a hospital pharmacist, the mother supervised employees manipulating cytostatic non-teratogenic substances, but she was given other tasks as soon as she planned to be pregnant. She complained of high professional stress during the first 16 weeks of gestation. She was not on any medication, did not drink alcohol, did not smoke (neither did her husband), and had no past medical or surgical history of note. She received regular prenatal care. She was seronegative for toxoplasmosis, syphilis, cytomegalovirus, and HIV, but seropositive for rubella. No infection or disease was reported during pregnancy.
The transverse congenital fetal lower limb anomalies were detected during ultrasonography at gestational age 22 weeks. Fetal ultrasound measurements were mainly between the 10th and 50th centiles (Table I), with no non-musculoskeletal finding. Therapeutic abortion, elected by the parents because of the lower limb abnormalities, was performed at 23 weeks and 2 days of gestation. At delivery, fetal weight was 420 g (9th centile) and external appearance was characterized by total absence of the zeugopodal and autopodal segments of both legs. Right and left thighs measured 4.5 cm and ended in folded buds of soft tissue 0.5 and 0.7 cm long, respectively, that resembled knee-like folds, especially on the left side (Fig. 1A–C). There was no other external anomaly and the placenta, membranes, and amniotic cavity were normal. There was no sign of an amniotic band or of any adherence of the membranes to the body.
| Parameters | Data | Reference values for 22WA fetus (P50) | Reference values for 22WA fetus (P10) |
|---|---|---|---|
| Occipito-frontal circumference (mm) | 193.0 | 196.4 | 188.0 |
| Biparietal diameter (mm) | 51.0 | 55.1 | 52.2 |
| Abdominal circumference (mm) | 162.0 | 172.8 | 161.4 |
| Right femoral length (mm) | 38.0 | 37.6 | 34.6 |
| Left femoral length (mm) | 36.0 | 37.6 | 34.6 |
| Right humeral length (mm) | 35.0 | 36.3 | 33.5 |
| Right radial length (mm) | 28.0 | 30.9 | 28.1 |
| Right ulnar length (mm) | 33.0 | 34.4 | 31.4 |
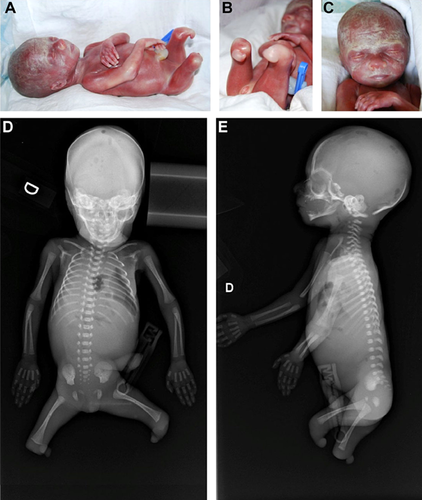
The absence of tibia, fibula, and all the foot bones was confirmed by radiographs. The number and shape of all other skeletal bones were normal (Fig. 1D and E). Estimated skeletal age corresponded to gestational age.
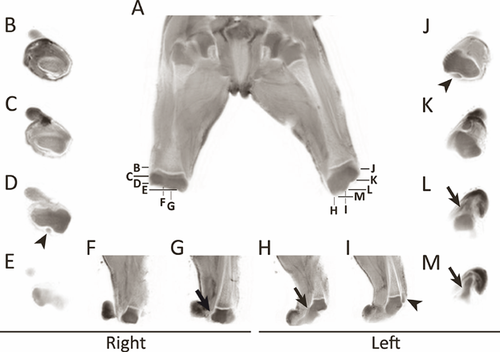
Magnetic resonance imaging (MRI) showed normal organization of the muscle compartments in the thigh (Fig. 2A). The patella was high (patella alta) in both limbs (Fig. 2D, I, and J: arrowheads). The distal femoral epiphyses appeared continuous with a short limb bud, which showed a signal similar to cartilage, without joint cavity (Fig. 2G, H, L, and M: arrows).
Internal examination of the fetus, including the skull, identified no other abnormality at autopsy. Histology of placenta, brain, lung, digestive tract, genitourinary system, and chondrocostal junction was normal, excluding recognizable syndromes.
Histological transverse sections showed that the right lower limb bud was composed of a cartilage-like mass surrounded by soft tissue (data not shown). Sagittal sections through the left lower limb bud were compared to sections through the lower limb of a healthy age-matched fetus aborted because of chorioamnionitis. The distal extremity of the left femur was composed of cartilage, as in the normal fetus (Fig. 3A: present fetus; 3B: normal fetus). However, it was continuous with a cartilaginous mass shaped like the proximal epiphysis of the tibia. A groove, observed at the presumed distal end of the femoral epiphysis, corresponded to the usual knee joint space, but there was no separation between femur and tibia (Fig. 3: arrows). The menisci and joint capsule were not visible. The patellar ligament was attached to the distal extremity of the limb bud instead of the tibial tuberosity (Fig. 3: L). Thigh vessels, nerves, and myocytes appeared normal. No particular condensation or aspect of the cells was seen in the area of the expected knee joint. The apex fibularis and the patella appeared normal and the thigh muscles had normal distal insertions (data not shown).

Fetal karyotype, analyzed on amniotic fluid, was normal (46,XX), whereas the mother and father were observed to be asymptomatic carriers, respectively, of an extension of the satellite stalk of chromosome 21 (46,XX,21pstk+) and of a pericentric inversion of chromosome 10 (46,XY,inv[10][p11.1q21.2]). Targeted sequencing of the exons of SHH, FGF4, FGF8, FGF10, HOXA10, HOXA11, HOXA13, HOXC10, HOXC11, HOXC12, HOXC13, HOXD10, HOXD11, HOXD12, and HOXD13 genes [Evans and Chudley, 1999; Goodman et al., 2000; Hermanns-Sachweh et al., 2005] was carried out on DNA extracted from the fetal scalp. Exome sequencing was performed on the trio (affected fetus and the two unaffected parents). We could not detect any damaging variant under de novo or recessive inheritance hypothesis.
All these investigations were performed at the parent's request, with their informed consent, and were considered part of clinical care by the Ethics committee of Université catholique de Louvain.
DISCUSSION
Although lower limb transverse anomalies are always associated with other anomalies [Frantz and O'Rahilly, 1961], the unique absence of both legs and feet can be classified according to the nomenclature outlined by Gold et al. [2011] as an isolated bilateral terminal transverse defect of the lower limbs with nubbins. In the present fetus, the nubbins consisted in cartilaginous buds in continuity with the femoral epiphyses.
Congenital malformations can be categorized according to their etiology: presumed vascular disruption anomalies (PVDA), teratogenic exposure, chromosomal abnormalities, genetic anomalies, known associations, or unknown etiology [Gold et al., 2011]. Although 20% can affect bilateral limbs, we could exclude a PVDA, such as the amniotic band syndrome, as the proximal segment of the limb buds featured cutaneous excess and the membranes were normal. The phenotype is unlikely to result from a teratogen as the mother was never in contact with drugs, such as 5-Aza-2′-Deoxycytidine, which can induce phocomelia or amelia [Rosen and Chernoff, 2002], or thalidomide [Kim and Scialli, 2011].
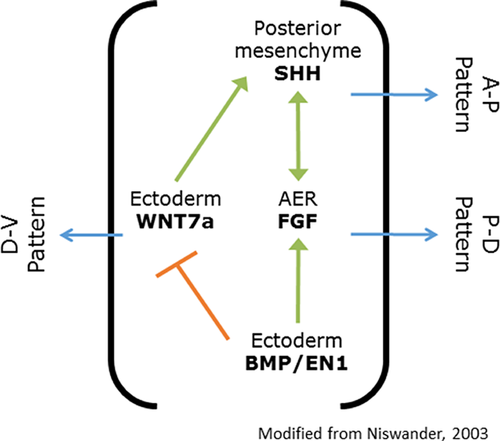
A progressive limb truncation was obtained in a stage-specific manner in chick wings [Summerbell, 1974] and lower limbs [Rowe and Fallon, 1982] by removing the apical ectodermal ridge (AER), which is involved in limb patterning (Fig. 4). The transverse limb anomalies observed in these chicks were comparable to the disruption of proximo-distal elongation of the lower limb observed in the present fetus. This latter condition, therefore, appears to be compatible with the AER theory, highlighting its potentially unique insight into the competing theories of limb morphogenesis. The Progress Zone theory proposes that the AER determines the fate of undifferentiated cells along limb elongation, the Early Specification Model proposes that the limb bud cells are specified, and that the AER stimulates their proliferation, avoiding apoptosis [Dudley et al., 2002]. Furthermore, lower and upper limb development appears to be associated, as there is no specific gene for lower limb development except for limb bud initiation (for review see [Duboc and Logan, 2011]).
The limb agenesis in the fetus described here challenges the current mechanistic theories of joint formation. As the chondrification of the proximal tibia corresponds to that of the distal femur at stage 22, joint interzone should be delineated by perichondrial tissue and menisci formation should occur consistently with joint cavitation [O'Rahilly and Gardner, 1975]. However, although the lower limb anlagen feature similar development, the knee interzone corresponds to stages 18–19, with mesenchymal cells present in the presumed joint cavity. This histological appearance argues for a developmental arrest before joint cavitation and suggests a molecular misregulation before interzone formation and mesenchymal differentiation [Pacifici et al., 2005]. This is of interest as the epigenetic regulation leading to joint cavitation is still unclear [Pacifici et al., 2005].
Particular attention was paid to chromosomal rearrangements as both parents had variant karyotypes. Although the father's pericentric inversion of chromosome 10 was not associated with clinical manifestations, 46,XY,inv(10)(p11q21) is known to be associated with congenital heart disease [Bugge et al., 2000] or chronic myeloid leukemia [Sun et al., 2011], whereas 46,XX,inv(10)(p11.1q21.1) has been linked to muscular disorders [Bugge et al., 2000]. Neither fetal karyotype nor the autopsy identified these anomalies. The pericentric inversion in p11 and q21 appears to be a weak point in chromosome 10 and has been associated with sterility or hypogonadism [Teyssier and Moreau, 1983]. Although related to familial inheritance, its cause is unknown [Kleczkowska et al., 1987]. Stressful life events around conception or during early gestation are currently classified as unknown etiology as they are reported to increase the risk of heart or neural tube anomalies, and cleft lip with or without cleft palate [Carmichael and Shaw, 2000; Hansen et al., 2000]. Although the mother complained of occupational stress, this single fetus with transverse lower limb anomaly is insufficient to support a stress-related etiology.
A genetic anomaly could not be excluded even without the evidence of a HOX, SHH, or FGF candidate gene mutation. In fact, this phenotype has not yet been described in either human or animal models in the medical literature (Table II). Hd homozygous mice manifest shortened distal toe phalanges [Hummel, 1970], Gli3 knockouts exhibit absent tibia and bowing fibula in the presence of Hoxd12 transgene [Chen et al., 2004], whereas Hoxc11 overexpressing mice have absent ulna and fibula [Papenbrock et al., 2000]. Hoxa11/Hoxd11 double knockout mice feature an almost total ulnar and radial loss [Davis et al., 1995], and transgenic mice expressing Hoxa9, Hoxd11, Hoxa13, and Hoxd13 using Prx1 promoter [Williams et al., 2006] have progressive shortening of the stylopod and/or zeugopod. In humans, genetic mutations in these genes are only associated with polydactyly [Guttmacher, 1993] or clinodactyly [Goodman et al., 2000], finger shortening [Innis et al., 2004], tibial agenesis [Evans and Chudley, 1999], fibular agenesis [Hermanns-Sachweh et al., 2005], or lower limb agenesis with [Chitayat et al., 1993] or without feet preservation [Sousa et al., 2008]. The genes listed in Table II did not show any damaging variant in the fetal DNA compatible with de novo or recessive mode of inheritance nor were we able to identify any other pathogenic variants in the exome data. Many of these candidate genes interact in the AER-related limb patterning (Fig. 4). The causative mutation may still lie in the non-coding parts of one of these genes, be a somatic mutation not detectable in the scalp DNA, or affect another, novel gene that was not identified in our analysis.
| Animal models | Human features | ||||
|---|---|---|---|---|---|
| Genes | Phenotypes | References | Genes | Features | References |
| Total limb agenesis | |||||
| Wnt3 | Total limb loss | Barrow et al. [2003] | WNT3 | Total limb loss | Niemann et al. [2004] |
| FGF4/8 | Total limb loss | Sun et al. [2002] | FGF8 | Long bone loss | Browne et al. [2012] |
| FGF10 | Total limb loss | Sekine et al. [1999] | FGF10 | Long bone loss | Browne et al. [2012] |
| Femur loss or hypoplasia | |||||
| Pitx1/2 | Femoral a- or hypoplasia | Marcil et al. [2003] | PITX1 | Tibial loss | Gurnett et al. [2008]; Klopocki et al. [2012] |
| Tbx4 | Femoral hypoplasia | Naiche and Papaioannou [2007] | TBX4 | Small patella | Bongers et al. [2004]; Kerstjens-Frederikse et al. [2013] |
| Hoxa10/Hoxc10/Hoxd10 | Femoral hypoplasia | Wellik and Capecchi [2003] | HOXA10/HOXC10/HOXD10(*) | Tibial loss | Evans and Chudley [1999] |
| Zeugopod loss or shortening | |||||
| Hoxa11/Hoxc11/Hoxd11 | Zeugopod shortening | Wellik and Capecchi [2003] | |||
| Shh Shh and Alx4 | Zeugopod shortening | te Welscher et al. [2002] | SHH | Tibial hemimelia | Cho et al. [2013] |
| SHH | Long bone absence | Browne et al. [2012] | |||
| ALX3/ALX4 | Tibial aplasia | Wechsler et al. [2004] | |||
| Tibia loss or shortening | |||||
| Pitx1/2 | Tibial a- or hypoplasia | Marcil et al. [2003] | PITX1 | Tibial loss | Gurnett et al. [2008]; Klopocki et al. [2012] |
| Wnt7a | Tibial loss or shortening | Parr and McMahon [1995] | WNT7 | Phalangeal and tarsal shortening | Woods et al. [2006] |
| HOXB | Tibial shortening | Maraia et al. [1991] | |||
| HOXD | Tibial/fibular hypoplasia | Dlugaszewska et al. [2006] | |||
| Fibula loss or shortening | |||||
| Tbx4 | Fibular shortening | Naiche and Papaioannou [2007] | TBX4 | Small patella | Bongers et al. [2004]; Kerstjens-Frederikse et al. [2013] |
| Hoxc11 | Fibular loss or shortening | Papenbrock et al. [2000] | HOXC10/11(*) | Fibular loss/aplasia | Hermanns-Sachweh et al. [2005] |
| Wnt7a | Fibular loss or shortening | Parr and McMahon [1995] | WNT7 | Phalangeal and tarsal shortening | Woods et al. [2006] |
| Autopod loss or alteration | |||||
| Shh and Alx4 | Autopod loss | te Welscher et al. [2002] | ALX4 | Brachydactyly | Verdyck et al. [2003] |
| Hoxa11/12/13 Hoxd11/12/13 | Digit loss | Zákány et al. [1997] | HOXD3/D13 | Tarsal, metatarsal bones, and toes loss | Del Campo et al. [1999] |
| HOXA13 | Tarsal bones shortening | Goodman et al. [2000]; Goodman and Scambler [2001] | |||
| HOXA13 | Metatarsal bones and toe loss or shortening | Innis et al. [2004] | |||
| Goodman et al. [2000] | |||||
| HOXD13 | Metatarsal bones and toe loss | Muragaki et al. [1996]; Goodman et al. [1998]; Goodman and Scambler [2001] | |||
| HOXD13 | Tarsal bones and toes shortening | Johnson et al. [2003] | |||
| HOXD13 | Phalangeal hypoplasia | Caronia et al. [2003] | |||
| HOXD13 | Brachydactyly | Goodman et al. [1997]; Zhao et al. [2007] | |||
| HOXD9-HOXD13 | Toe loss | Goodman et al. [2002] | |||
- Data are classified by phenotype severity from total limb loss to phalangeal shortening. (*) Suspected but unproven role.
In conclusion, we present a unique fetus with bilateral terminal transverse lower limb anomalies, with short nubbins at knee-level, in this case mesenchymal buds composed of a cartilage-like mass with muscular and dermal conservation. Further molecular analyses of the present fetus could yield clues concerning new factors and their possible interactions in limb genesis, which may ultimately lead to improvements in limb agenesis diagnosis.
ACKNOWLEDGMENTS
The authors thank Prof. Hélène Antoine-Poirel, Prof. Yves Sznajer, Miss Audrey Debue, and Mr Marc de Bournonville for their help and advice. We are grateful to Prof. Claire Craddock-de Burbure for revising the manuscript, and are deeply indebted to the parents of the fetus for their collaboration with this study.




