Thyroid nodules on chest CT of patients with tuberous sclerosis complex
Abstract
A few cases of thyroid disease have been reported in tuberous sclerosis complex (TSC); however, studies on prevalence and characterization of lesions have not been done. Patients with TSC are routinely screened using chest CT for assessment of lung disease. Incidental thyroid findings on chest CT have been reported in large studies of the general population. The purpose of this study is to evaluate the frequency and type of thyroid anomalies in a cohort of TSC patients. We performed a retrospective review of 93 patients with a definite diagnosis of TSC, who had a chest CT. Images of the thyroid gland and final radiological report were reviewed. Reports of additional thyroid studies performed in some patients were also reviewed. Thyroid abnormalities were present in 19 of 93 (20.4%) patients. They consisted mainly of hypodense lesions categorized as nodules. Multiple nodules were found in 10 patients (52.6%). There was one papillary carcinoma. Thyroid gland lesions may be part of the clinical spectrum of TSC. They are usually asymptomatic. As some cases of thyroid carcinoma have been described in TSC, ultrasound exams are recommended, given that CT is not the gold standard technique for thyroid evaluation. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder caused by mutations in TSC1 or TSC2, which result in hyperactivation of the mTOR pathway, influencing cell growth and neuronal cell migration. TSC is characterized by the development of hamartomas in several organs, particularly brain, lungs, heart, and kidneys [Crino et al., 2006]. There are several reports of thyroid disease in patients with TSC [Ghidini et al., 1971; Sareen et al., 1972; Wolff, 1973; Lie et al., 1980; Gomez, 1991; Bereket and Wilson, 1993; Jovic et al., 2001; Dicorato et al., 2009], but no studies determining its prevalence have been performed. Incidental thyroid findings on chest CT have been documented in large studies of the general population [Ahmed, 2012]. Our purpose was to evaluate the frequency of thyroid abnormalities detected on chest CT in patients with TSC.
MATERIALS AND METHODS
This retrospective study was compliant with HIPAA guidelines and was approved by the Institutional Review Board. Patients with a definite TSC diagnosis according to the current clinical diagnostic criteria [Roach et al., 1998] and at least one chest CT available for review were selected. According to our TSC protocol, scans were obtained with 0.74- to 5-mm-thick contiguous sections throughout the chest with or without contrast administration. Images and radiological report of the most recent chest CT available for all patients were reviewed. We recorded if the thyroid gland was partially or totally included in the scan. The presence of thyroid anomalies, including enlargement of the gland and the presence and laterality of nodules and calcifications, was recorded. When gathering the information in Table I, multiple nodules were noted when three or more nodules were visualized. When available, we also reviewed the report of thyroid sonographies. Information regarding pulmonary lesions was obtained from the radiological report; we considered multifocal micronodular pneumocyte hyperplasia (MMPH) when one or more nodules were reported. Demographic data and results of genetic testing for the overall cohort were recorded. For the sub-cohort of patients with thyroid abnormalities, the clinical phenotype (specifying major and minor features of TSC they present), and other features thought to be related to TSC that are not currently considered diagnostic criteria were also recorded. The clinical findings more frequent than expected in the group with thyroid involvement were compared to the group without thyroid involvement.
| P | G | Age (years) | Mutation | Thyroid lesions | Largest lesion (mm) | |
|---|---|---|---|---|---|---|
| 1 | F | 69 | TSC2 | Multiple L thyroid nodules | 15 | |
| R thyroid lobectomy (previous follicular adenoma) | ||||||
| 2 | F | 39 | TSC1 | One L thyroid nodule | L 20 | |
| Multiple R thyroid nodules | R 4 | |||||
| 3 | F | 57 | TSC1 mosaic | One R thyroid nodule | 4 | |
| Posterior R calcifications | ||||||
| 4 | M | 59 | TSC1a | One L thyroid nodule | 2 | |
| 5 | F | 27 | TSC1 | Multiple L thyroid nodules | 3 | |
| 6 | F | 48 | ND | Two L thyroid nodules | L 10 | |
| Two R thyroid nodules | ||||||
| 7 | F | 66 | NMI | Multiple bilateral nodules | 5 | |
| 8 | F | 65 | ND | One L thyroid nodule with calcification | 4 | |
| 9 | F | 28 | TSC2 mosaic | One L thyroid nodule | 3 | |
| 10 | F | 44 | TSC1 | One L thyroid nodule | 2 | |
| 11 | F | 57 | ND | One L thyroid nodule with fat and calcification | 4 | |
| 12 | F | 69 | TSC1 | One R thyroid nodule | 8 | |
| One R cyst | ||||||
| 13 | F | 64 | ND | Thyroidectomy (previous L papillary thyroid carcinoma) | – | |
| 14 | F | 53 | ND | Multiple bilateral nodules | 4 | |
| 15 | F | 35 | TSC2 | One L thyroid nodule | 3 | |
| 16 | F | 48 | TSC2a | Multiple bilateral nodules | 4 | |
| 17 | F | 48 | NMI | One R thyroid nodule | 2 | |
| 18 | F | 62 | ND | One R thyroid nodule | 2 | |
| 19 | F | 51 | NMI | Multiple bilateral thyroid nodules | 10 |
- F, female; G, gender; L, Left; M, male; ND, not determined; NMI, no mutation identified; P, patient; R, right.
- a Variant of unknown significance.
A total of 93 patients were identified (mean age 39 years; range 11–81 years), including 30 (32.3%) males and 63 (67.7%) females; 25 (26.9%) had a mutation in the TSC1, 29 (31.2%) in the TSC2, two of which had the TSC2/PKD1 contiguous gene syndrome (CGS) (2.2%), 16 (17.2%) had no mutation identified (NMI), three patients (3.3%) had variants of unknown significance (two in the TSC1 and one in the TSC2), and in 20 patients, (21.5%) genetic testing had not been performed. In 56 patients (60.2%), intravenous contrast was administered; in 55 CTs (59.1%), the thyroid could be completely seen; in 37 CTs (39.8%), it was incompletely seen, and in one (1.1%), it was not seen because of a prior thyroidectomy.
Statistical analyses were performed using SPSS version 11.5 for Windows. An alpha level of 0.05 was used for all statistical calculations. We applied chi-square and Fisher tests to analyze contingency tables.
RESULTS
Nineteen patients (20.4%) had thyroid abnormalities visible on chest CT; including one male and 18 females with a mean age of 52 years (range 27–69 years). Results for the 19 patients are summarized on Table I. Thirteen of the 19 patients had genetic testing, five (38.5%) had a mutation in the TSC1, three (23.1%) in the TSC2, three (23.1%) had NMI, and two (15.4%) had variants of unknown significance (one in the TSC1 and one in the TSC2). Eleven had a contrast-enhanced chest CT and the thyroid lesions were described as hypodense nodules (Fig. 1). In eight patients with unenhanced CT, the lesions were described as hypodense nodules, but they were less conspicuous than those on the enhanced studies (Fig. 1C). Two patients had calcifications and one (patient 11) had calcifications and areas of fat density, suggesting a possible angiomyolipoma. Postsurgical changes were identified in two: one patient (13) had undergone a previous total thyroidectomy because of a left papillary thyroid carcinoma, images prior to surgery were not available; the other patient (1) had had a right hemithyroidectomy due to a follicular adenoma. A retrospective review of the imaging reports in this patient showed that the lesion was evident 6 years prior to surgery as a heterogeneous hypodense lesion on the right thyroid lobule on an unenhanced CT.
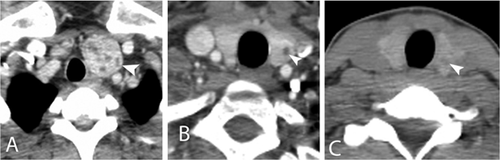
Multiple thyroid nodules were identified in 10 patients (52.6%), involving both lobes in seven; the right lobe only in two, and the left lobe only in one. Single nodules were seen in nine patients (47.4%); two in the right lobe and seven in the left lobe. Eighteen of 63 women (28.6%) had thyroid lesions, which were multiple in 10 (55.6%); and from all men (30), one (3.3%) presented a solitary thyroid lesion. Among patients with an enhanced CT (56 patients), thyroid lesions were identified in 11 (19.6%). The prevalence in females was of 28.9% (11 of 38 patients); no males had thyroid involvement on enhanced chest CT.
The thyroid lesions of 15 patients had been reported in the original radiological report and six were further studied with thyroid sonography. Four of the six had lesions that measured more than 10 mm; in one, it measured 8 mm; and from one, we did not have information available for review. In all, sonography showed more lesions than detected on the CT (Table II), and their characteristics were better described. The mean size of the lesions that were not further studied was 3.2 mm (range 2–5 mm).
| Chest CT | Sonography | FNA | Biopsy | Surgery | |
|---|---|---|---|---|---|
| Case 1 | R heterogeneous thyroid nodule (15 mm) (enlarged in serial CT) | One L cyst One R nodule | Non-diagnostic | Thyroid tissue with a microfollicular pattern | R thyroid lobectomy and isthmectomy Final diagnosis: Follicular adenoma |
| Case 2 | Enlarged L thyroid lobe Multiple L thyroid nodules (largest 20 mm) | One dominant L nodule Four R small nodule | – (Recommended after US; data not available) | – | – |
| Case 6 | One L thyroid nodule (10 mm) | Two L nodules Two R nodules (dominant L nodule enlarged on serial US) | – (Recommended after US; patient's decision not to do it) | – | – |
| Case 12 | Enlarged R thyroid lobe One R nodule (8 mm) | One R nodule One R cyst | – (Not recommended after US) | – | – |
| Case 19 | Multiple R thyroid nodules (largest 10 mm) | Three L nodules Four R nodules | Benign dominant R nodule | – | – |
- Patient 13 is not included because we had no available data for review.
- CT, computed tomography; FNA, fine-needle aspiration; L, left; R, right; US: ultrasound exam.
A majority of patients with thyroid lesions had TSC pulmonary involvement: 15 patients (78.9%) had MMPH and 12 (63.2%) had lymphangioleiomyomatosis (LAM). Among patients without thyroid abnormalities (74 patients; mean age 36 years, range 11–81 years), 50 patients (67.6%) had MMPH and 22 (29.7%) had LAM. There is a statistically significant (P = 0.00145) higher prevalence of LAM among patients with thyroid involvement. Prevalence of MMPH was slightly higher in this group, but this was not statistically significant (P = 0.4104). Results for the patients with and without thyroid involvement are summarized in Table III.
| Patients with normal thyroid (74 patients) | Patients with thyroid lesions (19 patients) | |
|---|---|---|
| Genotype | ||
| TSC1 mutation | 20 (27.0%) | 5 (26.3%) |
| TSC2 mutation | 26 (35.1%) | 3 (15.8%) |
| NMI | 13 (17.6%) | 3 (15.8%) |
| TSC1a | 1 (1.3%) | 1 (5.3%) |
| TSC2a | – | 1 (5.3%) |
| TSC2/PKD1 | 2 (2.7%) | – |
| Not tested | 14 (18.9%) | 6 (31.6%) |
| Lung disease | ||
| LAM | 22 (29.7%) | 12 (63.2%) |
| MMPH | 50 (67.6%) | 15 (78.9%) |
- LAM, lymphangioleiomyomatosis; MMPH, multifocal micronodular pneumocyte hyperplasia; NMI, no mutation identified; PKD, polycystic kidney disease.
- a Variant of unknown significance.
DISCUSSION
Thyroid disease in patients with TSC is presumed to be comparable to the general population, although hypothyroidism [Sareen et al., 1972], thyroid dysgenesis [Bereket and Wilson, 1993], goiter [Jovic et al., 2001], thyroid adenoma [Ghidini et al., 1971; Lie et al., 1980], thyroid papillary carcinoma [Ghidini et al., 1971; Wolff, 1973; Gomez, 1991], and one medullary thyroid carcinoma [Dicorato et al., 2009] have been reported. To our knowledge, no systematic studies about the prevalence of thyroid disease in patients with TSC have been performed, and surveillance for thyroid disease is not a part of the standard care in TSC [Roach et al., 1999]. Ultrasound is considered the gold standard for thyroid imaging.
In the general population, the prevalence of incidental findings on the thyroid gland varies depending on the mode of detection, it ranges from 2% to 6% on palpable examination, 9–67% using ultrasound, and 8–65% using autopsy data [Carroll, 1982; Woestyn et al., 1985; Ezzat et al., 1994; Steele et al., 2005; Bartolotta et al., 2006; Pinchera, 2007; Dean and Gharib, 2008], showing a female predominance and an increase with age [Carroll, 1982; Woestyn et al., 1985; Ezzat et al., 1994; Bartolotta et al., 2006; Olusola-Bello et al., 2013; Sharen et al., 2014]. Limited studies evaluating the frequency of incidental thyroid lesions detected on CT in the general population have been performed. Youserm et al. [1997] studied 123 patients with enhanced CT and 108 patients with MRI of the neck, finding an overall prevalence of 16% of thyroid abnormalities. Yoon et al. [2008] found a rate of 16.8% of incidental thyroid lesions in a cohort of 734 patients with enhanced neck CT. In a retrospective review of contrast-enhanced chest CTs of 2,510 adult subjects (1,222 women, 1,288 men), Ahmed [2012] found thyroid incidentalomas in 25.1%, which were more frequent in females (30.5%) than in males (19.9%). Our results show a similar overall prevalence of thyroid lesions in TSC patients with enhanced chest CT. Focusing on the female population (as in our study no males had lesions on enhanced chest CT), we find no difference in the prevalence of thyroid findings in women between both the studies (P = 1.0000).
Thyroid incidentalomas on chest CT usually present as solitary lesions (around 61.5% of all thyroid incidentalomas detected on CT are single) [Shetty et al., 2006; Ahmed, 2012]. In our series, nine patients (47.4%) had a single nodule and 10 (52.6%) had multiple lesions. Despite having a limited number of cases, our findings suggest a higher prevalence of multifocal involvement of the thyroid in TSC patients. This pattern was corroborated in six patients with thyroid ultrasound.
According to Shetty et al. [2006], the prevalence of malignancy of all thyroid incidental lesions detected on CT is about 3.9%, with a 7.4% rate of malignant potential. Ionizing radiation related to medical imaging increases the risk of developing cancer in general population [Brenner and Hall, 2007; Sodickson et al., 2009]. In particular, CT scans involving the thyroid gland performed under the age of 15 years have been reported to increase the risk of developing thyroid cancer [Schonfeld et al., 2011]. Although it has not been demonstrated in the adult population, risk related to prior radiation exposure should not be excluded [Pellegriti et al., 2013]. Additionally, patients with TSC might have an increased sensitivity to radiation as suggested by a study with an experimental animal model heterozygous for the TSC2 mutation, the Eker rat, that demonstrated an increase in the risk of renal cell carcinoma after radiation [Kokubo et al., 2010].
Our series include one case (patient 13) of papillary thyroid carcinoma. To our knowledge, only three other cases have been reported in patients with TSC [Ghidini et al., 1971; Wolff, 1973; Gomez, 1991]. Thyroid function, differentiation, and proliferation are mainly regulated by pituitary TSH through activation of the PI3K- mTOR pathway [Blancquaert et al., 2010], which has also been involved in the development of thyroid follicular neoplasms [Yeager et al., 2008; Miller et al., 2009]. Hence, dysregulation of the mTOR pathway in TSC [Crino et al., 2006] may provide a molecular substrate facilitating the appearance of thyroid nodules, as observed in other organs involved in this disease, including the lung (MMPH) or the brain (subependymal nodules).
Patients with thyroid findings correlated with a higher prevalence of LAM, these patients were older than those with normal thyroid (mean ages of 52 and 36 years respectively). A possible explanation could be that thyroid nodules increase with age [Popoveniuc and Jonklaas, 2012; Olusola-Bello et al., 2013; Russ et al., 2014; Sharen et al., 2014]; an association between LAM and older age has also been reported [Franz et al., 2001].
Prior studies found a prevalence of 18–22% for TSC1 mutations and 77–82% for TSC2 mutations among TSC patients [Jones et al., 1999; Sancak et al., 2005]. In our cohort with thyroid involvement, TSC1 mutations were more frequent than TSC2 mutations. However, this difference should not be taken into account, as there is an overrepresentation of women with a TSC1 mutation in our cohort.
Two patients had the TSC2/PKD1 CGS and presented with normal thyroid glands. Patients with the TSC2/PKD1 CGS have more severe renal disease [Brook-Carter et al., 1994; Cabrera-López et al., 2015] and cysts in the thyroid gland [Ul Haque and Moatasim, 2008]. Yet our findings do not demonstrate a higher incidence of thyroid affection in such patients.
It is uncertain how to handle an incidental thyroid lesion detected on CT. Because incidental thyroid findings cannot be reliably evaluated on CT, thyroid lesion detected with this technique regardless of its characteristics should be further studied with a thyroid ultrasound exam, as it is the best technique to stratify the malignancy of such lesions, adding subsequent explorations, such as fine-needle aspiration biopsy (FNA), when indicated [Liebeskind et al., 2005; Shetty et al., 2006; Jin and McHenry, 2012; Russ et al., 2014]. However, some authors suggest that not every lesion has to undergo an ultrasonographic exam and that other clinical features and risk factors, such as being larger than 1 cm, have to be considered [Ahmed et al., 2010]. Our study reinforces that ultrasound has a higher sensitivity in the detection of thyroid anomalies and provides greater detail.
The main limitation of our study is that we retrospectively reviewed CT of the chest performed for evaluation of suspected lung involvement. Knowing that CT underestimates the presence of thyroid pathology, prevalence of thyroid involvement in TSC is probably higher than our results suggest. Larger epidemiological studies should be conducted to evaluate the real prevalence of incidental thyroid involvement in patients with TSC and, ideally, thyroid ultrasound should be used.
In conclusion, our study shows a similar prevalence of incidental thyroid lesions on chest CT in patients with TSC compared with the general population. Due to the potential malignancy of thyroid incidental nodules [Shetty et al., 2006; Desser and Kamaya, 2008] and the fact that some TSC patients have multiple CT studies, a potential risk of developing thyroid carcinoma must be considered. We suggest that thyroid gland ultrasound evaluation are warranted in TSC patients showing thyroid incidental findings on CT. Still, larger epidemiologic studies should be conducted to clearly evaluate the actual prevalence of thyroid pathology in TSC.
ACKNOWLEDGMENTS
This work was supported by the Herscot Center for Tuberous Sclerosis Complex. S.B. and I.B. are supported by a BAE grant from the Carlos III Institute, Spain. The authors would like to thank Montserrat Bernat, M.D. and Anton Auladell, M.D. for their support.