A postzygotic NRAS mutation in a patient with Schimmelpenning syndrome
To the Editor
Schimmelpenning syndrome (OMIM 163200) is a rare neurocutaneous disorder characterized by craniofacial sebaceous nevus with central nervous system, ocular, or skeletal abnormalities. Sebaceous nevus presents as a yellowish, waxy skin lesion that can undergo neoplastic degeneration in 24% of patients [Moody et al., 2012]. The neurological abnormalities consist of developmental delay, seizures, and hemimegalencephaly [Davies and Rogers, 2002]. The ocular abnormalities include lipodermoids and coloboma. The skeletal abnormalities include incomplete formation of bony structures, underdeveloped bones, and short stature. Somatic mutations of HRAS and KRAS have been reported in patients with sebaceous nevus and Schimmelpenning syndrome [Groesser et al., 2012]. Activating HRAS and KRAS mutations are the underlying mechanism of the predisposition to adnexal skin tumors observed in patients with Schimmelpenning syndrome. Here, we report on a patient with Schimmelpenning syndrome and a postzygotic NRAS mutation.
The proposita was the first child of unrelated and healthy parents. She was delivered at 38 weeks of gestational age after an uneventful pregnancy. Her birth weight was 3,666 g (+1.6 SD), length 50.0 cm (+0.5 SD), and OFC 35.5 cm (+1.5 SD). At birth, she had yellowish skin lesions at the right frontal lobe and vertex, bilateral preauricular tags, capillary malformation of the midline face and chin, and a small congenital melanocytic nevus of the right face (Fig. 1). A skin biopsy from two lesions (Fig. 1, shown by arrows) demonstrated sebaceous nevus with abortive hair follicles and acanthosis in both lesions. No melanocytic nevus cells were seen in the biopsy tissue of the patient. She developed a strawberry hemangioma of the right postauricular skin. An ocular examination showed retinal coloboma and a small right macula. Cranial magnetic resonance imaging (MRI), T2-weighted coronal images showed a large right temporal lobe with a polymicrogyric cortex suggesting hemimegalencephaly, and T1-weighted axial images showed small fat signal masses in the bilateral cerebellopontine angle (Supplemental Figure S1). Bone X-ray was normal. She achieved head control at 4 months and began crawling and walking with support at 16 months. She developed seizures at the age of 10 months and carbamazepine was administered. After the administration of antiepileptic drugs, she had complex partial seizures with impaired consciousness and dysconjugate gaze approximately twice daily. Electroencephalography detected a spike in the right occipital lobe.
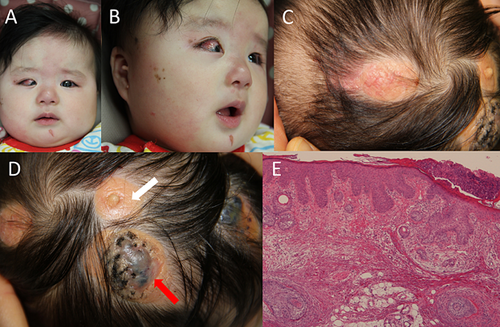
Total genomic DNA was obtained from the patient's nevus (shown by white arrow) and lymphocytes using standard techniques. PCR products of exon 1 of HRAS were sequenced directly in the nevus sample. The patient's samples from the nevus and lymphocytes were sequenced using MiSeq (Illumina, Inc., San Diego, CA) with 121 bp and 150 bp paired-end reads after enrichment with TruSight Tumor and SureSelect Human Kinome DNA kits, respectively. The TruSight Tumor kit (Illumina, Inc.) was designed for targeted next-generation deep sequencing of the following 26 oncogenes and tumor suppressor genes, covering all coding regions (total 21 kb): AKT1, ALK, APC, BRAF, CDH1, CTNNB1, EGFR, ERBB2, FBXW7, FGFR2, FOXL1, GNAQ, GNAS, KIT, KRAS, MAP2K1, MET, MSH6, NRAS, PDGFRA, PIK3CA, PTEN, SMAD4, SRC, STK11, and TP53. The SureSelect Human Kinome DNA kit (Agilent Technologies, Inc., Santa Clara, CA) was designed for 612 kinase genes, oncogenes, and related genes, covering all coding regions (total 3.2 Mb). Data were analyzed by MiSeq Reporter (Illumina, Inc.) and displayed in the Integrative Genomics Viewer. Mutations identified by panel sequencing with the nevus and lymphocytes were confirmed by Sanger sequencing and segregated appropriately in lymphocytes.
No mutation was detected by direct sequencing of HRAS exon 1 and panel sequencing of KRAS exon 2 in the nevus, where previous mutations have been found in Schimmelpenning syndrome [Groesser et al., 2012]. Targeted deep sequencing (average coverage of 15,896) identified an exon 2 NRAS mosaic mutation (c.182A>G, which predicts p.Q61R) in the nevus sample. The mutant allele frequency was 30.6% (6,913/22,590 reads). Direct sequencing demonstrated the decreased mutant peak (Supplemental Figure S2). The NRAS mutation could not be detected in the lymphocytes by panel sequencing (read depth 187) and direct sequencing, suggesting that the mutation was postzygotic.
We reported on a patient with Schimmelpenning syndrome and a postzygotic NRAS mutation in codon 61. Somatic activating mutations of HRAS and KRAS have been reported in patients with sebaceous nevus and Schimmelpenning syndrome; however, an NRAS mutation has not been detected previously in a patient with sebaceous nevus [Groesser et al., 2012]. RAS promotes cell growth through the activation of the mitogen-activated protein kinase (MAPK) signal transduction pathway. Germline mutations of the genes in the RAS-MAPK pathway cause Noonan syndrome and related disorders, which are referred to as RASopathies. Activating germline HRAS mutations cause Costello syndrome, which is characterized by multiple congenital anomalies, developmental delay, cancer predisposition, hyperkeratosis, and cutaneous papillomas [Aoki et al., 2005]. This finding suggests that the activation of the downstream RAS-MAPK signaling pathway plays a crucial role in the pathogenesis of sebaceous nevus and neoplastic development.
The NRAS p.Q61R mutation identified in this patient has been reported as a recurrent mutation in patients with congenital melanocytic nevus (CMN) syndrome [Kinsler et al., 2013; Charbel et al., 2014]. The CMN syndrome presents as a neurocutaneous disorder with giant multiple CMNs and neurological abnormalities including the brain parenchyma, hydrocephalus, arachnoid cysts, syringomyelia, and brain tumors. The CMN syndrome is also associated with an increased risk of melanoma transformation. According to the COSMIC database, this mutation (COSM584) was also detected in malignant melanomas and benign melanocytic nevus. In malignant melanoma, NRAS mutations of codon 61 play pivotal roles in tumor genesis and maintenance through the activation of the MAP/ERK cascade pathway [Eskandarpour et al., 2005]. NRAS mutations in CMN also cluster at codon 61, indicating that activating mutations in the RAS-MAPK signaling pathway lead to CMN with other anomalies and the predisposition to adnexal cutaneous neoplasia being inherent in sebaceous nevus and Schimmelpenning syndrome. Co-occurrence of sebaceous nevus syndrome and giant CMN has been reported in four patients with no genetic study [Mimouni et al., 1986; Lam et al., 2008; Hsieh et al., 2012]. Lam et al. [2008] described these conditions as SCALP syndrome (Sebaceous nevus syndrome, Central nervous system malformations, Aplasia cutis congenita, Limbal dermoid, and Pigmented nevus [giant congenital melanocytic nevus]). The present patient had multiple sebaceous nevi and a small melanocytic nevus, but not aplasia cutis congenita. The clinical features in this patient are more consistent with Schimmelpenning syndrome than SCALP syndrome. Sebaceous nevus and CMN would share a common etiology associated with mosaic RASopathy.
In conclusion, we identified the first causative NRAS mutation in Schimmelpenning syndrome. Accordingly, postzygotic NRAS mutations can cause both CMN and Schimmelpenning syndrome.
ACKNOWLEDGMENTS
This research was supported in part by a Grant-in-aid from the Ministry of Health, Labor, and Welfare, Japan. We thank the patient and her family for their cooperation.




