The diagnostic value of next generation sequencing in familial nonsyndromic congenital heart defects
Abstract
To determine the diagnostic value of massive parallel sequencing of a panel of known cardiac genes in familial nonsyndromic congenital heart defects (CHD), targeted sequencing of the coding regions of 57 genes previously implicated in CHD was performed in 36 patients from 13 nonsyndromic CHD families with probable autosomal dominant inheritance. Following variant analysis and Sanger validation, we identified six potential disease causing variants in three genes (MYH6, NOTCH1, and TBX5), which may explain the defects in six families. Several problematic situations were encountered when performing genotype-phenotype correlations in the families to confirm the causality of these variants.
In conclusion, by screening known CHD-associated genes in well-selected nonsyndromic CHD families and cautious variant interpretation, potential causative variants were identified in less than half of the families (6 out of 13; 46%). Variant interpretation remains a major challenge reflecting the complex genetic cause of CHD. 2015 Wiley Periodicals, Inc.
INTRODUCTION
Isolated CHD are generally thought to have a multifactorial origin, though in a small proportion of cases, estimated at 4%, there is a clear familial recurrence [Oyen et al., 2009; Oyen et al., 2010; van der Bom et al., 2011], suggesting a single genetic cause. The inheritance pattern is typically autosomal dominant with variable expression and reduced penetrance. More rarely, autosomal recessive or X-linked inheritance exists. Traditional cloning approaches have led to the identification of a large number of genes for nonsyndromic CHD [Schott et al., 1998; Garg et al., 2003; Garg et al., 2005; Kirk et al., 2007], but each of these genes is implicated only in a small proportion of familial CHD. Genes associated with CHD can be found in CHDWiki [Barriot et al., 2010] (http://www.esat.kuleuven.be/∼bioiuser/chdwiki).
Traditional genetic diagnosis of a large panel of candidate genes suffers from limitations in sequencing capacity. Nowadays, this is overcome by next-generation sequencing (NGS) technologies, which enable large-scale DNA sequencing. Selective enrichment followed by massive parallel sequencing of the genomic regions of interest has been widely applied in genetic research, and is gradually being implemented in clinical diagnostic settings [Ellard et al., 2013; Mook et al., 2013; Wang et al., 2013].
MATERIALS AND METHODS
Families and Clinic Phenotypes
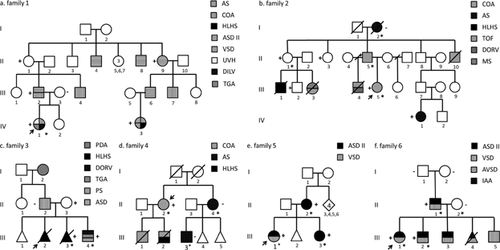
Thirteen families with multiple affected individuals with CHD were recruited from the genetic and cardiology clinics in three University Hospitals in Belgium (Leuven 11, Ghent 1 and Louvain-la-Neuve 1). Inclusion criteria were families with a nonsyndromic CHD in at least three first or second degree relatives. The type of CHD needed to be confirmed by cardiac ultrasound, and the predominant heart defect in the family is known to be etiologically heterogeneous (thus excluding e.g. families with supravalvular aortic stenosis, known to be exclusively caused by ELN gene mutations). Unaffected individuals included in the study were examined by cardiac ultrasound. Moreover, DNA from at least one affected individual was available. Informed consent was obtained from all participants or their legal representative. The study conforms to the principles outlined in the Declaration of Helsinki, and was approved by the local ethical committee of the UZ Leuven (S52853). The pedigrees of the families are shown in Figure 1 and Supplementary Figure 1. Genomic DNA was extracted from peripheral white blood cells in all individuals.
Probe Design
We selected 57 genes (Table I) known to harbor pathogenic variants in CHD cases based on the literature and CHDWiki (January 2012). Our main interest was in nonsyndromic CHD genes. However, since many syndromes have variable expressivity, we also included syndromic CHD genes. The custom capture arrays or in-solution probe libraries were synthesized by NimbleGen (Roche NimbleGen, Inc., Madison, WI), including repeat-masked sequences of all exons of all possible transcripts with 50 bp intronic and 1 kb promoter region sequences. The tiling probes cover above 92% bases in the 523 kb target region.
| Gene | Phenotype correlation | OMIM | Genomic coordinates (GRCh37/hg19) |
|---|---|---|---|
| Nonsyndromic | |||
| ACTC1 | ASD | 102540 | chr15:35,080,296–35,087,926 |
| ACVR2B | Heterotaxy/PS | 602730 | chr3:38,495,789–38,534,632 |
| ALDH1A2 | TOF | 603687 | chr15:58,245,621–58,358,120 |
| ANKRD1 | TAPVC | 609599 | chr10:92,671,857–92,681,032 |
| CFC1 | TOF/TGA/IAA | 605194 | chr2:131,350,334–131,357,081 |
| CITED2 | ASD/VSD | 602937 | chr6:139,693,395–139,695,784 |
| CRELD1 | AVSD | 607170 | chr3:9,975,523–9,987,096 |
| ELN | PPS/SVAS/AS | 130160 | chr7:73,442,426–73,484,236 |
| FOXC2 | HLHS | 602402 | chr16:86,600,856–86,602,538 |
| FOXH1 | TOF/TGA | 603621 | chr8:145,699,114–145,701,717 |
| FOXL1 | HLHS | 603252 | chr16:86,612,114–86,615,303 |
| GATA4 | ASD/PS/TOF/VSD | 600576 | chr8:11,561,716–11,617,508 |
| GATA6 | ASD/PTA/PS/PDA | 601656 | chr18:19,749,415–19,782,226 |
| GDF1 | Heterotaxy/TGA/TOF | 602880 | chr19:18,979,360–19,006,952 |
| HAND1 | ASD/VSD/AVSD/TOF/HLHS | 602406 | chr5:153,854,533–153,857,824 |
| LEFTY2 | Heterotaxy/TGA/AVSD | 601877 | chr1:226,124,297–226,129,082 |
| MCTP2 | COA | NA | chr15: 94,774,767–95,027,180 |
| MED13L | TGA | 608771 | chr12:116,396,380–116,714,990 |
| MYH11 | PDA/TAAD | 160745 | chr16:15,796,991–15,950,886 |
| MYH6 | ASD/Tricuspid atresia | 160710 | chr14:23,851,198–23,877,485 |
| MYH7 | Ebstein's anomaly of tricuspid valve/ASD | 160760 | chr14:23,881,946–23,903,495 |
| NKX2-5 | ASD/VSD/TOF/HLHS | 600584 | chr5:172,659,106–172,662,314 |
| NKX2-6 | PTA | 611770 | chr8:23,559,963–23,563,921 |
| NODAL | Heterotaxy/TOF | 601265 | chr10:72,191,691–72,201,464 |
| NOTCH1 | BAV/LVOTO | 190198 | chr9:139,388,895–139,440,237 |
| PDGFRA | TAPVC | 173490 | chr4:55,095,263–55,164,411 |
| SMAD6 | BAV/AS/COA | 602931 | chr15:66,994,673–67,074,337 |
| TAB2 | BAV/LVOTO | 605101 | chr6:149,639,435–149,732,746 |
| TBX20 | ASD/VSD/MS | 606061 | chr7:35,242,041–35,293,710 |
| TDGF1 | TOF/VSD | 187395 | chr3:46,616,044–46,623,952 |
| ZFPM2 | TOF | 603693 | chr8:106,331,146–106,816,766 |
| ZIC3 | Heterotaxy/TGA/PS | 300265 | chrX:136,648,345–136,654,258 |
| Syndromic | |||
| JAG1 | Alagille syndrome/TOF | 601920 | chr20:10,618,331–10,654,693 |
| NOTCH2 | Alagille syndrome | 600275 | chr1:120,454,175–120,612,316 |
| TBX5 | Holt-Oram syndrome | 601620 | chr12:114,791,734–114,846,246 |
| TBX3 | Ulnar-mammary syndrome | 601621 | chr12:115,108,058–115,121,968 |
| TBX1 | DiGeorge syndrome/TOF | 602054 | chr22:19,744,225–19,771,115 |
| TFAP2B | Char syndrome | 601601 | chr6:50,786,438–50,815,325 |
| SHOC2 | Noonan syndrome | 602775 | chr10:112,723,882–112,773,424 |
| PTPN11 | Noonan/LEOPARD syndrome | 176876 | chr12:112,856,535–112,947,716 |
| KRAS | Noonan/Cardiofaciocutaneous syndrome | 190070 | chr12:25,358,179–25,403,853 |
| SOS1 | Noonan syndrome | 182530 | chr2:39,208,689–39,347,603 |
| RAF1 | Noonan/LEOPARD syndrome | 164760 | chr3:12,625,099–12,705,699 |
| BRAF | Noonan/Cardiofaciocutaneous syndrome | 164757 | chr7:140,433,811–140, 624,563 |
| MAP2K1 | Cardiofaciocutaneous syndrome | 176872 | chr15:66,679,210–66,783,881 |
| MAP2K2 | Cardiofaciocutaneous syndrome | 601263 | chr19:4,090,318–4,124,125 |
| EHMT1 | Kleefstra syndrome | 607001 | chr9:140,513,443–140,730,578 |
| RAI1 | Smith-Magenis syndrome | 607642 | chr17:17,584,786–17,714,766 |
| SALL4 | Duane-radial ray syndrome | 607343 | chr20:50,400,580–50,419,047 |
| MLL2 | Kabuki syndrome | 602113 | chr12:49,412,757–49,449,106 |
| HRAS | Costello syndrome | 190020 | chr11:532,241–535,549 |
| NSD1 | Sotos syndrome | 606681 | chr5:176,560,079–176,727,213 |
| NIPBL | Cornelia de Lange syndrome | 608667 | chr5:36,876,860–37,065,920 |
| SMC1A | Cornelia de Lange syndrome | 300040 | chrX:53,401,069–53,449,617 |
| SMC3 | Cornelia de Lange syndrome | 606062 | chr10:112,327,448–112,364,391 |
| CHD7 | Charge syndrome | 608892 | chr8:61,591,323-61,780,586 |
| ZEB2 | Mowat-Wilson syndrome | 605802 | chr2:145,141,941–145,277,957 |
Targeted Massive Parallel Sequencing
Library construction for all samples followed the TruSeq DNA Sample Preparation Protocol using Illumina DNA sample preparation kits (Illumina, Inc., San Diego, CA). 4 or 5 indexed samples were pooled for one hybrid capture. All exons of the target genes were captured through hybridization with custom designed nucleotide probes, by either NimbleGen Sequence Capture 385K Arrays (on-slides) or, in the second part of the study by NimbleGen SeqCap EZ Choice library (in-solution). The target-enriched libraries were amplified and sequenced by the Illumina HiSeq2000 paired-end sequencing.
Sequencing Data and Variant Analysis
Bioinformatics processing of the sequencing data was done using the GATK pipeline. The paired-end reads were mapped against the human reference genome hg19 using BWA (0.6.2). GATK UnifiedGenotyper (2.4–9) was used for variant calling. Annovar (11-02-2013) was used for functional annotation of detected variants.
The inheritance pattern in the families was compatible with an autosomal dominant pattern with incomplete penetrance, we thus looked for shared variants in affected members and obligate carriers in each family. In Family 12, autosomal recessive inheritance was also considered. Small insertions, deletions, and single nucleotide variants (SNVs) affecting the coding exons or splicing sites of the target genes were considered as candidates. Since the pathogenic variants are supposed not to be frequent in the normal population, we used the 1,000 genomes project and the ESP database as reference to filter out variants with a minor allele frequency (MAF) above 1% in the human population. Synonymous variants were also filtered out. The effects of nonsynonymous variants were predicted by SIFT, PolyPhen2 and MutationTaster. Candidate variants in each family were validated by Sanger sequencing. The identified certain or likely causative variants were submitted to ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/) and dbSNP (http://www.ncbi.nlm.nih.gov/snp) databases.
RESULTS
Targeted Massive Parallel Sequencing
Approximately 70% of the sequences were uniquely aligned to the reference genome, ∼92% target bases were covered, and ∼89% target bases were covered with a sequencing depth above 30X. When checking the canonical transcript, 20 exons in 18 genes (Supplementary Table I) were not well covered (sequencing depth < 30X). Most of them are the first exons containing UTR sequences with high GC content, which undergo difficulties during amplification. The coding regions of the 20 exons with low sequencing depth were resequenced by Sanger sequencing.
Variant Analysis
After filtering a total of 45 variants were identified in the 36 patients from 13 families (Supplementary Table II). The number of identified variants ranges from 0 to 6 per patient. Of these 45 variants, six variants presented in multiple families with different phenotypes and did not segregate with the defect in the families. Since they also presented in an in-house exome database, they were either rare local variants in the Belgian population or technical artifacts. One other variant (rs200520088) was confirmed to be false positive by Sanger sequencing. Among the remaining 38 variants, 26 were present in either the 1,000 genome program database or the ESP database with a MAF below 1% (Supplementary Table II). These are thus rare variants, present in the normal population. All of them are missense variants, of which only two are predicted as damaging by all three in silico prediction programs, but they do not segregate with the disease in the family. For the other 12 unique variants, genotyping in 1,000 local controls was performed using Sequenom MassARRAY Platform (Sequenom, Inc., San Diego, CA). Two of them were local rare variants (Supplementary Table II). Taking into account the functional impact, six variants (five novel and one reported) in three genes were identified as functional deleterious variants, which are probably responsible for the defects in six families (Table II, Fig 1). The other six variants were either with non-pathogenic prediction by at least one out of three prediction programs (SIFT, PolyPhen2 and MutationTaster) or did not segregate with the disease in the family, or both. Identified variants in all available family members in the six families were validated by Sanger sequencing.
| Family | Phenotype | Nr of sequenced | Detected variants |
|---|---|---|---|
| 1 | ASD/VSD | 1 | MYH6:NM_002471.3:c.2033A>G,p.Asn678Ser |
| 2 | HLHS/COA/AS/MS | 4 | NOTCH1:NM_017617.3: c.5281del, p.Arg1761Glyfs*37 |
| 3 | DORV/TGA/PS/HLHS | 2 | NOTCH1:NM_017617.3: c.2014+1G>A |
| 4 | COA/AS/HLHS | 3 | NOTCH1:NM_017617.3: c.5061G>T, p.Gln1687His |
| 5 | ASD/VSD | 3 | TBX5:NM_000192.3:c.709C>T,p.Arg237Trp (rs104894328) |
| 6 | ASD/VSD | 4 | TBX5:NM_000192.3:c.301A>T,p.Ile101Phe NOTCH1:NM_017617.3:c.5332G>C,p.Ala1778Pro |
| 7 | PDA | 3 | MLL2:NM_003482.3:c.10192A>G,p.Met3398Val (rs75937132) NOTCH1:NM_017617.3:c.4129C>T,p.Pro1377Ser (rs61751542) |
| 8 | TOF | 2 | NOTCH1:NM_017617.3:c.6685G>A,p.Val2229Met (rs202096917) ZEB2:NM_014795.3:c.2141C>T,p.Pro714Leu (rs112581563) |
| 9 | PTA/TOF | 2 | TBX5:NM_080718.1:c.998C>T,p.Pro333Leu (rs28730762) |
| 10 | AS | 4 | – |
| 11 | AS | 3 | – |
| 12 | PDA | 3 | – |
| 13 | ASD | 2 | – |
- Pathogenic variants probably responsible for the defects were identified in six families (marked in bold)
In Family 1 (Fig 1a), multiple members have atrial septal defect type secundum (ASD II) and/or perimembranous ventricular septal defect (VSD). Two patients have complex cardiopathies. The proband (IV1) has a functional univentricular heart, a dominant double-inlet right-sided morphologically left ventricle and rudimentary left-sided morphologically right ventricle, stenosis of the left atrial-ventricular valve, a VSD with a mild degree of subaortic stenosis and L-transposition of the great arteries (TGA). Her female second cousin (IV3) has a hypoplastic left heart syndrome (HLHS) with a small mitral valve, a malaligned perimembranous VSD causing subaortic stenosis, small left ventricular outflow tract and aorta, and a hypoplastic aortic arch with coarctation. She also has a fenestrated atrial septum. A novel missense variant in MYH6 (c.2033A>G, p.Asn678Ser) was detected, which segregates with the disease in all affected individuals investigated. The variant alters a highly conserved amino acid in the myosin head domain, and is predicted to be functional deleterious by three different in silico prediction programs (SIFT: 0, Polyphen2: 0.978, MutationTaster: disease causing).
In Family 2 (Fig 1b) with variable left ventricular outflow tract obstructions (LVOTO), a heterozygous 1bp frameshift deletion (c.5281del, p.Arg1761Glyfs*37) was identified in NOTCH1. The deletion results a premature stop codon, truncating the majority of the Notch1 intracellular domain. Sanger sequencing confirmed the deletion in Patients II5, III5 and IV1, also in three unaffected family members including obligate carrier II1, indicating an incomplete penetrance. The deletion was not carried by Patient I2, who was previously diagnosed with age-related degenerative aortic valve stenosis (AS).
In Family 3 (Fig 1c), two siblings (III2, III3) were diagnosed with HLHS, for which the pregnancies were terminated respectively. Their brother (III4) has double outlet right ventricle (DORV), TGA (type not specified) and pulmonary stenosis (PS). The father (II2) has ASD II, and the paternal grandmother (I2) had patent ductus arteriosus (PDA). We identified a splice donor site variant (c.2014 + 1G>A) in NOTCH1 in the sister and the brother of the proband, inherited from the mother.
In Family 4 (Fig 1d), the severity of the CHD ranges from AS, coarctation of the aorta (COA) to HLHS. A novel missense variant in NOTCH1 (c.5061G>T, p.Gln1687His) was identified in the proband (II2) who had COA. This variant changes a highly conserved amino acid in the Notch_NODP domain, with pathogenic in silico predictions (SIFT: 0.01, PolyPhen2: 0.997, MutationTaster: disease causing). According to HGMD (professional 2013.4), no variant in this domain of NOTCH1 has been described previously. Of interest, this variant was not present in her sister and niece (II4, III3), who presented with AS.
In Families 5 (Fig 1e) and 6 (Fig 1f), presenting ASD and/or VSD, variants were identified in TBX5: in Family 5 a previously reported pathogenic variant (c.709C>T, p.Arg237Trp), and in Family 6 a novel missense variant (c.301A>T, p.Ile101Phe) with pathogenic in silico predictions (SIFT: 0, PolyPhen2: 0.948, MutationTaster: disease causing). The p.Arg237Trp variant in Family 5 was carried by all three affected patients (II2, III1 and III3). The p.Ile101Phe variant in Family 6 alters a highly conserved amino acid in the DNA-binding T-box domain, which occurred de novo in Individual II1, and was transmitted to the two affected siblings (III1 and III2). An additional novel variant was identified in NOTCH1 (c.5332G>C, p.Ala1778Pro) in the three affected members of Family 6, with non-pathogenic in silico predictions.
DISCUSSION
Given the genetic heterogeneity, genetic testing for familial nonsyndromic CHD is challenging. The exceptions are families with defined CHD with a single known causative gene, such as ELN and SVAS/PPS [Urban et al., 2000] or NKX2-5 in families with ASD associated to AV conduction defects [Gutierrez–Roelens et al., 2002]. The present results show that in a significant proportion of well-selected familial CHD (6 out of 13; 46%), a causative variant can be identified using the newest sequencing technology, allowing the massive parallel resequencing of all known cardiac genes in one single assay, which thus holds a value as a diagnostic test. In this study, pathogenic variants were only detected in three genes, TBX5, NOTCH1 and MYH6. Of interest, pathogenic variants in the same genes were detected in a recent report using a similar approach as the present study [Blue et al., 2014]. This suggests that in future, a smaller gene panel might be established, taking advantage of the parallel analysis of several genes commonly causing familial CHD, and thus avoiding the burden of numerous variants of unknown significance.
Given the enormous amount of human genetic variation, we anticipated and encountered interpretational difficulties of the found variants. Restricting the analysis to a well-defined panel of validated genes for CHD facilitated the interpretation. In total, using stringent filtering criteria (as described in Materials and Methods section), only 45 potential functionally significant variants were found in these 13 families. In the families where a functional deleterious variant was found, the interpretational challenges remain in assigning causality to these variants.
In all families where a certain or likely causative variant was identified, the found gene matched the previously reported genotype-cardiac phenotype associations. For instance, in Family 1, we identified a MYH6 missense variant. MYH6 has been described as the predominant sarcomeric disease gene for familial ASD, and particularly, perturbations in the MYH6 head domain is thought to be the major genetic cause for familial ASD [Posch et al., 2011]. However, association of mutations in MYH6 with TGA has previously only been reported once in a mutation analysis study of MYH6 in patients with a wide spectrum of sporadic CHD [Granados–Riveron et al., 2010]. In three families with LVOTO (Families 2, 3, and 4), we identified three functional deleterious variants in the NOTCH1 gene: a frameshift deletion, a splice site variant and a missense variant. Notch signaling plays an important role in cardiac outflow tract development [de la Pompa and Epstein, 2012]. Missense mutations in NOTCH1 have been found in bicuspid aortic valve (BAV) and left ventricular outflow tract malformations including AS, COA, and HLHS [McBride et al., 2008], NOTCH1 haploinsufficiency resulted from nonsense or frameshift mutations is thought to cause familial aortic valve disease [Garg et al., 2005]. However, missense variants with pathogenic in silico predictions sometimes can be found in normal population. For this reason, without further functional studies, it is difficult to confirm the causality of the missense variant found in Family 4. In Families 5 and 6, two missense variants were identified in TBX5. Mutations in TBX5 are known to cause Holt–Oram Syndrome, which is characterized by skeletal malformations in upper limbs and cardiac defects, most commonly ASD and VSD [Mori and Bruneau, 2004]. Clinically the affected members in these two families present as isolated CHD, without observable limb defect. This is of interest, since TBX5 mutations typically are associated with limb defects, which can be variable. For instance, Cross et al. [2000] reported a family with very mild limb defects in only one affected individual. The fact that TBX5 mutations can be associated with apparently isolated CHD is also supported by the recent finding by Blue et al. [2014] who detected a TBX5 mutation in two families with isolated CHD. A recent study by Baban et al. [2014] also reported TBX5 mutations in patients with isolated tetralogy of Fallot (TOF). This indicated that screening of a larger, unbiased panel of genes might lead to novel genotype–phenotype correlations. Previously, targeted gene testing was only performed for families with suggestive features, for instance, heart-limb anomalies in the case of TBX5 testing [McDermott et al., 2005].
Genetic testing aims to improve genetic counseling in familial CHD. At first sight, counseling for CHD appears to be straightforward. However, in most families, even though the inheritance pattern is autosomal dominant, there is reduced penetrance and variable expressivity, which complicates risk determination. For instance, in Family 2, several clinically unaffected individuals (besides the obligate carriers) were shown to be carriers. They have thus an increased risk for affected offspring. Moreover, the high incidence of CHD raises the possibility of phenocopies. Indeed, in two of the families, we encountered affected individuals who did not carry the found variant. In Family 2, Individual I2, who had an AS, did not carry the familial NOTCH1 variant. It is uncertain if the patient had congenital abnormalities of the aortic valve, but given her advanced age, this would most likely fit with the more common age-dependent degenerative aortic valve stenosis. The identified novel frameshift deletion in NOTCH1 is probably associated with the defect in the family. NOTCH1 mutations are known to cause a spectrum of aortic valve anomalies, which fit with the phenotypes in Family 2. The frameshift variant was not found in 1,000 normal controls, and is thought to have a high functional impact by resulting in a truncated protein. Finding the same variant in a replication cohort would provide further validity. In Family 4, two sisters present a LVOTO, but only in the sister with COA, a NOTCH1 variant is implicated. This is unexpected, since it is commonly assumed that AS, COA, and HLHS are part of a spectrum of CHD, with shared pathogenesis and etiology. One explanation is that the found NOTCH1 missense variant may not be causal in this family. Alternatively, the two sisters may carry a different variant. The two affected members with AS carried a same missense variant in EHMT1 (c.752C>G, p.Pro251Arg), which alters a conserved amino acid and is predicted to be pathogenic (SIFT: 0, PolyPhen2:0.995, MutationTaster: disease causing). Mutations in EHMT1 are known to cause Kleefstra Syndrome, an autosomal dominant disorder characterized by severe intellectual disability and characteristic dysmorphic features. However, neither patient showed any manifestation of the syndrome, and this variant was also detected in a control person (1/1000), indicating the results by in silico prediction programs need to be interpreted with caution. This also indicates the value of studying as many affected family members as possible in order to evaluate the significance of found variants.
Familial CHD is characterized by remarkable variability in expression. One hypothesis is that this is related to additional genetic and/or environmental modifiers. Thus, it is possible that additional rare variants in one or more of the other CHD-associated genes cause a more complex or severe CHD in affected individuals. For this reason, we have searched for genetic modifiers in the gene panel we investigated. For instance, the MYH6 missense variant identified in Family 1 explains the prevailing phenotype (ASD II) in the family, but the proband also has TGA, which is a distinct phenotype from other family members, and there is no clear genotype–phenotype correlation between MYH6 and TGA. We hypothesized that besides the MYH6 variant explaining the common defects in the family, a variation in another gene could contribute to the additional phenotype. However, we did not identify in this individual any novel or rare variant in any of the genes associated with TGA, including CFC1, NODAL, ZIC3, MED13L, ACVR2B, GDF1, and LEFTY2 [Goldmuntz et al., 2002; Muncke et al., 2003 ; Chhin et al., 2007; Mohapatra et al., 2009; D'Alessandro et al., 2013].
In seven families, no functional deleterious variant was detected in the analyzed genes. There are a number of possible explanations. First, there are technical issues. Incomplete capture of targets may occur because repetitive regions are removed during the tiling probe design, to ensure the uniqueness of the capturing. Certain regions of our targets were excluded due to the presence of a pseudogene or highly similar sequences in other genomic regions. In addition, during the library preparation, genomic regions with very high GC content undergo difficulties in amplification, resulting in reduced or no sequencing coverage. Although the paired-end deep sequencing strategy sometimes enables sequencing of poorly covered parts of the targets, ∼10% of total targets were not well sequenced, where less than 30 reads passing the QC criteria aligned. However, coding sequences of the canonical transcripts of target genes account for only ∼ 6% of not well sequenced region.
Second, our analysis focused on variations in exons and exon–intron boundaries of the target genes exclusively, known to harbor the majority of monogenic mutations. Variants disrupting regulatory elements located in deep introns, UTR or intergenic regions thus remain undetected. A recent study shows possible pathogenic effect of variations disrupting cardiac enhancers located upstream of SOX9 [Sanchez–Castro et al., 2013].
Third, current NGS data processing methods are limited in detecting genomic structural variants from targeted sequencing data. Structural variants have been implicated in the pathogenesis of CHD, partial gene deletions or duplications disrupting gene function are rare causes of familial CHD. For instance, chromosomal translocations or deletions disrupting the elastin gene can cause familial supravalvular aortic stenosis and supravalvular pulmonary stenosis [Morris, 1998]. A TBX5 intragenic duplication was indicated to cause atypical Holt–Oram syndrome phenotype in a family [Patel et al., 2012]. Our analysis pipeline did not include tools to detect structural variants.
Fourth, in those families where no causative variant was detected, a novel gene may be implicated. Novel genes have been constantly reported in association with CHD. Since the design of this capture panel, mutations in GATA5, BVES, and GJA5 were shown to cause sporadic or familial TOF [Guida et al., 2013; Wei et al., 2013; Wu et al., 2013], MEF2C mutations were identified in patients with outflow tract defects [Kodo et al., 2012], NPHP4 and DICER1 variants were described in association with TGA [Foulkes et al., 2011; French et al., 2012].
Finally, CHD is genetically heterogeneous: a same phenotype can result from alterations in different genes, while different variations interrupting the same gene can lead to different phenotypes. Due to the complexity of the genotype–phenotype correlation of CHD, the monogenic model may not apply to all nonsyndromic CHD families.
In conclusion, although the genetic heterogeneity of CHD and remarkable variability of expression make it challenging to interpret the large number of identified variants, setting up a well-defined analysis pipeline enables effective identification of causative variants. Application of targeted massive parallel sequencing in clinical diagnosis represents a break-through for genetic testing of (familial) nonsyndromic CHD and other complex diseases with high genetic heterogeneity. However, difficulties assigning causality with certainty of the found variants is the main challenge. Nevertheless, in over half of our CHD families with obvious genetic factors, no potential causative variant was identified by screening a panel of known CHD associated genes, revealing a large proportion of unknown genetic causes of familial CHD.
ACKNOWLEDGMENTS
This study was supported by KU Leuven [GOA/12/015] and EU FP7 Programme “CHeartED” [HEALTH-F2-2008-223040].
We thank the patients and their families who participated in this study.




