Patients with isolated oligo/hypodontia caused by RUNX2 duplication
Abstract
Loss-of-function mutations of RUNX2 are responsible for cleidocranial dysplasia, an autosomal dominant disorder characterized by delayed closure of cranial sutures, aplastic or hypoplastic clavicles, moderate short stature and supernumerary teeth. By contrast, an increased gene dosage is expected for duplication of the entire RUNX2 sequence and thus, a phenotype different from cleidocranial dysplasia. To date, two cousins with a duplication including the entire RUNX2 sequence in addition to MIR586, CLIC5 and the 5' half of SUPT3H have been reported. These patients presented with metopic synostosis and hypodontia.
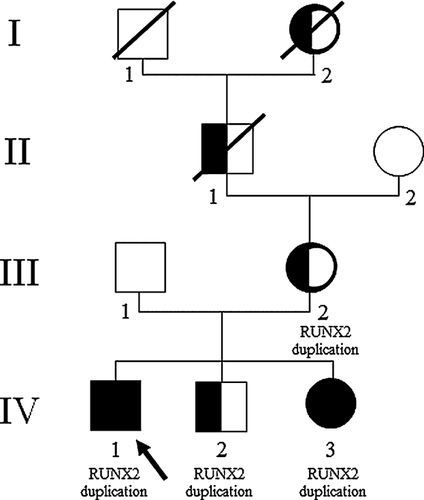
Here, we report on a family with an affected mother and three affected children. The four patients carried a 285 kb duplication identified by array comparative genomic hybridization. The duplication includes the entire sequence of RUNX2 and the 5′ half of SUPT3H. We confirmed the duplication by real-time quantitative PCR in the four patients. Two children presented with the association of metopic craniosynostosis and oligo/hypodontia previously described, confirming the phenotype caused by RUNX2 duplication. Interestingly, the mother and one child had isolated hypodontia without craniosynostosis, broadening the phenotype observed in patients with such duplications. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Runt-related transcription factor 2(RUNX2; OMIM *600211) is a key regulatory gene in intramembranous bone formation. Loss-of-function mutations of this gene and deletions including RUNX2 have been identified in patients presenting with cleidocranial dysplasia (CCD, OMIM #119600), an autosomal dominant disorder including delayed closure of cranial sutures, aplastic, or hypoplastic clavicles, moderate short stature and supernumerary teeth [Lee et al., 2008; Ryoo at al., 2010; Chen et al., 2013; Lee et al., 2013]. Conversely, partial intragenic duplications of the gene have been described in patients presenting with metaphyseal dysplasia with maxillary hypoplasia and brachydactyly (MDMHB; OMIM 156510) [Halal et al., 1982; Moffatt et al., 2013; Avela et al., 2014], whereas complete duplications have been described in patients presenting with craniosynostosis and oligodontia [Mefford et al., 2010; Greives et al., 2013]. Both types of duplication are acting as gain-of-function mutations.
Here, we report on the phenotype of four patients from a single family. We identified by array Comparative Genomic Hybridization (aCGH) a duplication including the entire sequence of RUNX2 and the 5' half of SUPT3H.
CLINICAL REPORT
Patient III-2
Patient III-2 is the mother of Patients IV-1, IV-2, and IV-3 (Fig 1). She presented with isolated dental agenesis, as did her father (II-1) and paternal grandmother (I-2). She had no dysmorphic features. Height was 150 cm (−2 SD) and weight 55.5 kg (within the normal range).
Patient IV-1
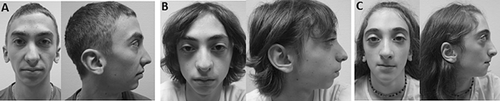
This male patient was born to nonconsanguineous healthy parents at 39 weeks gestation. At birth, his measurements were: weight 2,760 g (<10th centile), height 51 cm (within the normal range) and OFC 35 cm (within the normal range). Craniosynostosis was initially suspected because of mild turricephaly, but favorable outcome was observed without surgical care. At 11 years, height was 127.5 cm ( + 0.5 SD), weight 27.4 kg (−1 SD), and OFC 53.5 cm (−0.5 SD). Motor and cognitive development was normal. Walking was acquired at 18 months of age. Cryptorchidism was surgically corrected at 4 years. Physical examination revealed mild lumbar scoliosis. At the age 17 years 9 months, he was referred for hypodontia (agenesis of teeth 12, 22, 24, and 35) and radiculomegaly (teeth 17, 14, 34, and 46). Height was 163 cm (−2 SD), weight 49 kg (−2 SD), and OFC 55 cm (−1 SD). He presented with dysmorphic features including facial asymmetry, large eyebrows and synophrys, malar hypoplasia, low set ears with posterior angulation, and micrognathia (Fig 2A). Hands and feet were of normal size, but we noticed unilateral single transverse palmar crease, bilateral hallux valgus and partial 2–3 syndactyly of the feet. He had a dermatographism and brisk reflexes. Skeletal survey did not show abnormalities.
Patient IV-2
This male patient was born at 34 weeks gestation, with growth parameters within the normal range: birth weight 1,960 g (25th centile), birth height 42 cm (10th centile) and OFC 31 cm (30th centile). Two seizures at the ages 6 and 9 months led to anticonvulsive therapy with sodium valproate until he was 3 years old. Walking was acquired at 12 months. Development was marked by stereotypy, hyperactivity, and sleeping disturbances. Normal cognitive development and high intelligence quotient were suggestive of Asperger syndrome. At the age 8 years 8 months, height was 121 cm (−1 SD), weight 19.4 kg (−2.5 SD), and OFC 52 cm (−1.5 SD). He presented with dysmorphic features including large eyebrows, malar hypoplasia, low set ears with posterior rotation, and micrognathia (Fig 2B). Hand examination showed unilateral single transverse palmar crease. Dental examination revealed two missing teeth (left maxillary canine and one inferior incisors) and microdontia of 12 and 22. Skeletal survey did not show abnormalities.
Patient IV-3
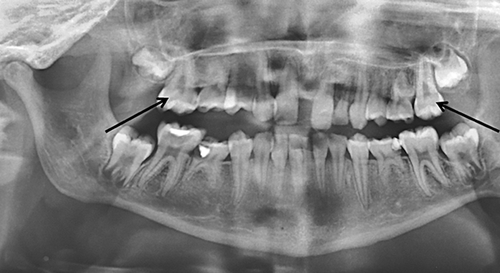
This female patient was born at 36 weeks gestation, with low growth parameters: birth weight 1,950 g (4th centile), height 44 cm (12th centile) and OFC 29 cm (below 1st centile). She presented with a mild turricephaly, but favorable outcome was observed without surgical care. At the age 4 years 9 months, height was 104.5 cm (within the normal range), weight 14.3 kg (−2 SD), and OFC 49.5 cm (+0.5 SD). Motor and cognitive development was normal, except for speech difficulties and dyslexia. She walked at 12 months. As her oldest brother, she was referred at 11 years 7 months for oligodontia (agenesis of teeth 15, 13, 12, 22, 23, 25, 33, 35, 43, and 45) and taurodontism (16 and 26) (Fig 3). Height was 143 cm (within the normal range), weight 28 kg (−2 SD), and OFC 51 cm (−1 SD). She presented similar dysmorphic features as Patient IV-2 (Fig 2C). She had several episodes of painful swelling of hands and feet, which required use of a wheelchair. Skeletal survey did not show abnormalities (Supplementary Figures 1 and 2 in supporting information online).
MOLECULAR CYTOGENETIC STUDY
Informed consent for genetic analyses was obtained from the patients' parents according to local ethical guidelines. Genomic DNA was extracted from peripheral blood using standard protocols. Molecular karyotyping in Patient IV-1 was initially conducted using a 60 K Agilent array with the ISCA design (http://www.iccg.org/) according to manufacturer's instructions. Array was analyzed with an Agilent scanner and the Feature Extraction software (v. 10.5.1.1). Graphical overview was obtained using the Genomic Workbench software (v.5.0).
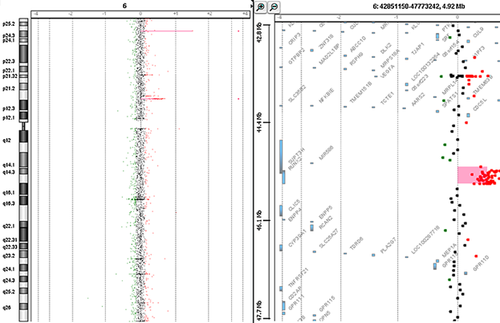
We identified in Patient IV-1 a 285 kb duplication localized on chromosome 6 (arr[hg19] 6p21.1(45,128,523 × 2,45,233,225–45,518,790 × 3,45,595,945 × 2) encompassing the whole coding sequence of RUNX2 and 60 kb upstream sequence of the gene and the 5′ half of SUPT3H (Fig 4). We confirmed the duplication in Patient IV-1 by real-time quantitative PCR (qPCR) using the ΔΔCt method [Livak et al., 2001], which consisted of comparing the Cts of reference genes (ALB and SULF1) with Cts of two targets located in RUNX2 (primers available upon request). Familial analysis performed by qPCR showed that the duplication was maternally inherited and present in his brother, IV-2, and his sister, IV-3.
DISCUSSION
Few duplications involving RUNX2 have been described. The first was detected by qPCR and reported by Ott et al. [2010] in a patient presenting with CCD. The duplication involved at least exons 1–4, but the exact size of the duplication remains unknown in the absence of additional molecular cytogenetic studies. Since the patient presented with CCD, the duplication is likely responsible for RUNX2 loss-of-function. However, in the absence of additional analyses performed on the product of the duplication (mRNA or protein), it remains unproven.
An intragenic RUNX2 duplication has also been reported in a family of French Canadian origin. The patients had a different condition called metaphyseal dysplasia with maxillary hypoplasia and brachydactyly (MDMHB) associating short stature, long bone, and spinal abnormalities, and dystrophic teeth [Moffatt et al., 2013]. The enlargement of the medial halves of the clavicle seen in this syndrome could represent a “mirror” sign to aplastic or hypoplastic clavicle in CCD. The identified intragenic RUNX2 duplication encompassed exons 3–5 and led to the production of a protein variant p.[Pro21_Arg229dup;Arg229Asn] which contains two QA domains and two Runt domains instead of one, and the substitution of an evolutionary conserved amino acid residue (Arg229). The authors suggested a gain-of-function effect and showed in vitro an increased transcriptional activity that strengthened this hypothesis. Recently, a second patient with MDMHB, not of French Canadian origin, has been reported [Avela et al., 2014]. Molecular characterization showed that exons 3–5 were duplicated, but the exact boundaries of the duplication were different from the first family. Since the consequences on RUNX2 transcript were identical to those in the first family, the authors concluded that the MDMHB phenotype results from an intragenic duplication of RUNX2 exons 3–5.
Intragenic duplications, leading to loss-of-function of RUNX2 causing the CCD phenotype or to gain-of-function responsible for the MDMHB phenotype, should be distinguished from a duplication of the entire RUNX2 sequence, as in our report. The first report that we consider as a “mirror” phenotype to CCD concerned a 1.1 Mb inverted duplication including the entire sequence of RUNX2, MIR586, CLIC5 and the 5′ half of SUPT3H. This duplication was identified by Mefford et al. [2010] in two affected cousins presenting with the metopic craniosynostosis and hypodontia. A similar clinical phenotype was reported by Greives et al. [2013] with a 1.24 Mb triplication at the same region (the exact boundaries of the duplication were not indicated in their report).
Here we described the smallest duplication encompassing the whole coding sequence of RUNX2 and its promoting region that likely results in increased gene expression and increased production of the wild type protein. Among the four patients reported herein, two (IV-1 and IV-3) present a syndromic oligo/hypodontia, and two (III-2 and IV-2) present isolated hypodontia. The clinical presentation shown by syndromic Patients IV-1 and IV-3 appears radically different from MDMHB, but shares a few similarities with previously duplication patients, including as hypodontia and craniosynostosis. Isolated hypodontia observed in Patients III-2 and IV-2 highlights a wider clinical spectrum associated with RUNX2 duplication, in addition to isolated and syndromic craniosynostosis and MDMHB.
ACKNOWLEDGMENTS
We thank the patients and their family for their contribution to this work. We are most grateful to the Genomics platform of Nantes (Biogenouest Genomics) core facility for its technical support.