Phenotype–genotype correlations in 17 new patients with an Xp11.23p11.22 microduplication and review of the literature
Abstract
Array comparative genomic hybridization (array CGH) has proven its utility in uncovering cryptic rearrangements in patients with X-linked intellectual disability. In 2009, Giorda et al. identified inherited and de novo recurrent Xp11.23p11.22 microduplications in two males and six females from a wide cohort of patients presenting with syndromic intellectual disability. To date, 14 females and 5 males with an overlapping microduplication have been reported in the literature. To further characterize this emerging syndrome, we collected clinical and microarray data from 17 new patients, 10 females, and 7 males. The Xp11.23p11.2 microduplications detected by array CGH ranged in size from 331 Kb to 8.9 Mb. Five patients harbored 4.5 Mb recurrent duplications mediated by non-allelic homologous recombination between segmental duplications and 12 harbored atypical duplications. The chromosomal rearrangement occurred de novo in eight patients and was inherited in six affected males from three families. Patients shared several common major characteristics including moderate to severe intellectual disability, early onset of puberty, language impairment, and age related epileptic syndromes such as West syndrome and focal epilepsy with activation during sleep evolving in some patients to continuous spikes-and-waves during slow sleep. Atypical microduplications allowed us to identify minimal critical regions that might be responsible for specific clinical findings of the syndrome and to suggest possible candidate genes: FTSJ1 and SHROOM4 for intellectual disability along with PQBP1 and SLC35A2 for epilepsy. Xp11.23p11.22 microduplication is a recently-recognized syndrome associated with intellectual disability, epilepsy, and early onset of puberty in females. In this study, we propose several genes that could contribute to the phenotype. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Intellectual disability (ID) occurs in 1–3% of the population and may be syndromic or nonsyndromic [Frints et al., 2002]. The role of chromosomal abnormalities as the main genetic etiology became particularly clear with the advent of array comparative genomic hybridization (array CGH) which enabled the detection of submicroscopic imbalances in approximately 12% of patients with ID and/or congenital malformations. Regarding the X chromosome, several cases of pathogenic copy number variants, corresponding to microduplications, have been reported especially among males. The anomaly can result either from an intra-chromosomal rearrangement, from an inter-chromosomal rearrangement between the two X chromosomes or from an unbalanced translocation between an X chromosome, an autosome, or a Y chromosome. Common rearrangements are Xq28 duplication involving MECP2, Xq21q22 duplication involving PLP1, Xp11.22 duplication involving HUWE1 and Xp11.23p11.22 [Froyen et al., 2012, 2008; Regis et al., 2008; Giorda et al., 2009; Ramocki et al., 2010]. Usually, X chromosome rearrangements are identified in males and inherited from a healthy mother. Indeed, most female carriers show severely skewed X inactivation of the rearranged X chromosome and are thus asymptomatic. However, women carrying X chromosomal rearrangements may present clinical features if there is a random X chromosome inactivation, a skewed X-inactivation of the normal chromosome, or the presence of genes escaping from X-inactivation [Dibbens et al., 2008]. In 2009, Giorda et al. identified inherited and de novo recurrent Xp11.23p11.22 microduplications in two males and six females from a wide cohort of patients presenting with syndromic ID [Giorda et al., 2009]. To date, 14 females and 5 males with a microduplication overlapping this region have been reported in the literature [Froyen et al., 2007; Bonnet et al., 2006; Edens et al., 2011; El-Hattab et al., 2011; Flynn et al., 2011]. The observed phenotype might include moderate to severe ID with usually severe language impairment, abnormal electroencephalogram with or without seizures, overweight, and early onset of puberty [Giorda et al., 2009]. Most of these patients harbored the same 4.5 Mb Xp11.23p11.22 microduplication generated by non-allelic homologous recombination (NAHR) between the paralogous distal (D-REP) and proximal (P-REP) repeat sequences. Here, we report on 17 new patients, 10 females and 7 males, with Xp11.23p11.22 microduplication (5 patients with the 4.5 Mb recurrent duplication and 12 patients with an atypical duplication) detected by array CGH. Atypical microduplications allowed us to define minimal critical regions that could be responsible for specific clinical findings of the syndrome and to highlight some genes: FTSJ1 and SHROOM4 for intellectual disability, PQBP1 and SLC35A2 for epilepsy, and BMP15 for early onset of puberty.
MATERIALS AND METHODS
Patients
A total of 17 patients (10 females and 7 males) recruited from the genetics department of Necker-Enfants Malades hospital and the French array CGH platforms network (http://www.renapa.univ-montp1.fr/) were included in this study. All patients had a microduplication that overlapped at least partially with the common 4.5 Mb Xp11.23p11.22 interval [Giorda et al., 2009]. Epileptic syndromes were determined from the recently redefined classification of the International League Against Epilepsy [Berg et al., 2010]. Informed consent for genetic testing was obtained from all tested patients and their relatives.
Molecular Cytogenetics
Different array platforms were used for genomic copy number analyses which were carried out according to manufacturers' recommendations: Agilent CGH Microarray 44 K and 60 K (Agilent Technologies, Santa Clara, CA) and Perkin Elmer Microarray 5.2 K (Waltham, MA). Chromosomal rearrangements were confirmed by fluorescence in situ hybridization (FISH) with various specific probes on chromosome preparations from leukocyte cultures or by quantitative polymerase chain reaction (PCR) using standard protocols. Parental testing was performed when DNA samples were available. Genomic positions are relative to human genome GRCh37/hg19.
X-Inactivation Study
For seven patients (Patients 1–4, 8–10) and three asymptomatic mothers (Patients 11–12, 14–16, and 17), the X chromosome inactivation (XCI) pattern was determined from peripheral leukocytes by PCR analysis of a polymorphic CAG trinucleotide repeat located in the polyglutamine region of the AR (androgen receptor) gene [Allen et al., 1992]. The XCI pattern was defined as skewed when cut-off values of the XCI ratio were ≥75:25 (moderately skewed inactivation) or ≥90:10 (severely skewed). The 50:50 cut-off value was defined as random.
RESULTS
Array CGH Results
All 17 patients harbored duplications overlapping the Xp11.23p11.22 region (Fig. 1, Supplementary File 1 in supporting information online) and ranging in size from 331 Kb to 8.9 Mb. In female patients, these duplications ranged from 4.5 Mb to 8.9 Mb. Five females harbored the 4.5 Mb recurrent duplication with breakpoints in the D-REP and P-REP duplicons. In four females, the size of the duplicated segments ranged from 4.5 Mb to 6.13 Mb, extending on either side of the 4.5 Mb recurrent duplication. One female (Patient 9) had a large 8.9 Mb duplication that extended to the band Xp11.3. The duplications identified in the seven male patients were smaller, ranging in size from 331 Kb to 4.4 Mb.
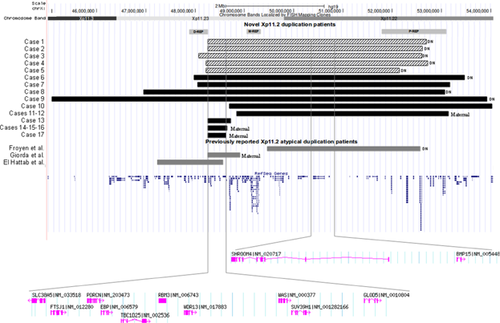
One of the shortest region of duplication overlap (SRO) was delineated in Patient 17, with estimated breakpoints at genomic positions chrX:48,317,353–48,648,283 (Fig. 1). No other pathogenic CNVs were identified in this patient. This 331 Kb segment includes 11 RefSeq genes: SLC38A5 (solute carrier family 38, member 5), FTSJ1 (FtsJ homolog 1), TBC1D25 (TBC1 domain family, member 25), PORCN (porcupine homolog), EBP (emopamil binding protein), RBM3 (RNA binding motif (RNP1, RRM) protein 3), WDR13 (WD repeat domain 13), WAS (Wiskott–Aldrich syndrome), SUV39H1 (suppressor of variegation 3–9 homolog 1), GLOD5 (glyoxalase domain containing 5), and GATA1 (GATA binding protein 1). Another region of interest of 24 Kb was located at genomic positions chrX:48,750,710–48,774,953, Hg 19, including four RefSeq genes: TIMM17B (translocase of inner mitochondrial membrane 17 homolog B (yeast)), PQBP1 (polyglutamine binding protein 1), SLC35A2 (solute carrier family 35 (UDP-galactose transporter), member A2), and PIM2 (pim-2 oncogene) (Supplemental data file 2 - see supporting information online). All patients presenting with epilepsy had a duplication overlapping this region except for patient 17 in whom the centromeric breakpoint was located approximately 102 Kb away. Parental samples were available for inheritance testing in 11 of the 14 families reported in this study. The anomaly occurred de novo in eight female patients (Patients 1–6, 8–9). Maternal inheritance was identified in six male patients from three families: Patients 11–12 with a 4.4 Mb duplication, Patients 14–16 with a 351 Kb duplication, and Patient 17 with a 331 Kb duplication.
Clinical Features of Patients
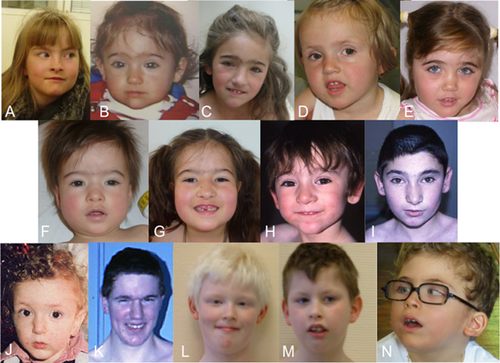
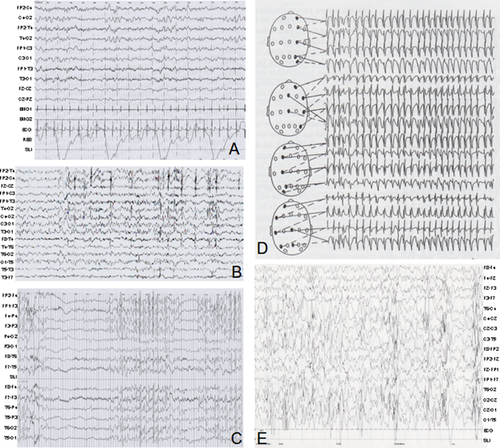
Phenotypic data for all 17 patients are summarized in Table I. Photographs of Patients 1, 3–5, 8, 11–12, 14–15, and 17 are provided in Figure 2. Epileptic phenotypes are further detailed in Table II for Patients 2–13, 15, and 17 and electroencephalograms (EEGs) are shown in Figure 3 for Patients 3, 9, and 13. The present study also provides a review of the 19 previously reported patients, including 13 with the 4.5 Mb recurrent microduplication. Thus, clinical data from 36 patients (including our 17 patients), 12 males, and 24 females, from 29 different families were collected and summarized in Table III. Clinical findings are summarized separately for the 18 patients with the 4.5 Mb recurrent microduplication (Table III).
| Gender | DN/m | Size | Skewed X inactivation (>75:25) | Age (year) | Psychomotor delay | Language impairment | Intellectual disability | Epilepsy | Brain MRI | Sleep disorder | Behavior disorder | Abnormal neurological examination | Genital anomaly | Abnormalities of extremities | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | DN | 4.5 Mb | + (≥90/10) | 9.5 | – | + | IQ 75 | – | Night terrors | Agressive behavior, attention deficit | – | Early onset of puberty | – | |
| 2 | F | DN | 4.5 Mb | – | 12 | + | + | Mild (cognitive decline) | Tonic-clonic seizures at 11 years | + | Agressive behavior | – | Early onset of puberty | – | |
| 3 | F | DN | 4.5 Mb | – | 9 | + | + | Mild | – | Normal | + | Attention deficit | – | Early onset of puberty | + |
| 4 | F | DN | 4.5 Mb | + (10/90) | 4.5 | + | + | Moderate | – | Normal | + | – | – | + | |
| 5 | F | DN | 4.5 Mb | nt | 6 | + | + | Moderate | – | Normal | – | – | Hypotonia, ataxia, pyramidal syndrome | + | |
| 6 | F | DN | 5.53 Mb | nt | 28 | + | + | Severe | Focal seizures at 23 years | Moderate ventricular dilatation | – | Hyperactivity, hyperphagia, Aggressive behavior | – | Early onset of puberty | + |
| 7 | F | 5.13 Mb | nt | 18 | + | + | Severe | Infantile spasms then generalized, absence and atonic seizures | Normal | – | – | Hypotonia | Early onset of puberty | – | |
| 8 | F | DN | 6.13 Mb | + (80/20) | 6 | + | + | Moderate | Focal seizures at 4 ½ months | Normal | – | Low frustration tolerance | Hypotonia, spasticity | – | |
| 9 | F | DN | 8.9 Mb | + | 10 | + | + | Profound | Infantile spasms at 9 months | Non specific white matter lesions | + | Central axis stereotypies, automutilation | Hypotonia | Early onset of puberty | – |
| 10 | F | 5.4 Mb | + | 9.5 | + | + | Severe | Febrile seizures at 7 months and 3 years, myoclonic seizures | Thick corpus callosum | – | Attention deficit, impulsiveness | – | Early onset of puberty | – | |
| 11 | M | m | 4.4 Mb | − (a) | 16 | + | + | Severe | – | Cerebral atrophy | Night laughs | – | Hypotonia | – | + |
| 12 | M | 12 | + | + | Severe | – | Normal | Night laughs | Hyperactivity | – | Shawl scrotum | – | |||
| 13 | M | 454 Kb | nt (a) | 9 | + | + | Moderate | Infantile spasms at 5 months | Ventricular dilatation, hypoplasia of corpus callosum | Night terrors | Aggressive behavior | – | Hypospadias | – | |
| 14 | M | m | 351 Kb | + (≥90/10) (a) | 10 | + | + | Severe | – | Frontal-temporal cortical atrophy | – | – | – | – | – |
| 15 | M | 9 | + | + | Severe | – | Normal | – | – | – | – | – | |||
| 16 | M | 3 | + | + | Severe | – | Normal | – | – | Intentional tremor, fine motor trouble | – | – | |||
| 17 | M | m | 331 Kb | + (a) | 2 | + | + | Severe | Infantile spasms at 6 months | Moderate myelinisation delay | – | Angry outbursts | – | Hypospadias, micropenis, cryptorchidism | – |
- dn: De Novo, F: Female, Iq: Intellectual Quotient, M: Male, M: Maternally Inherited, Mri: Magnetic Resonance Imaging, nt: Not Tested.
- a X inactivation was performed on carrier mother for these male patients.
| Age of onset | Type of seizures | Electroencephalograms | Epilepsy follow-up | Intellectual disability | Brain MRI | |
|---|---|---|---|---|---|---|
| 2 | 11 years | Generalized tonic- clonic seizures | 9 years : Continuous Spikes Waves during Sleep (CSWS) 13 years : normal background, right frontal-temporal spikes in awakeness slightly activated by sleep. | Seizure free on Valproate | Mild, moderate cognitive regression | – |
| 3 | – | – | 5 years : normal background, right anterior spikes-waves in awakeness, CSWS7 years : normal background, high amplitude right anterior spikes and spikes-waves activated during sleep. | CSWS controlled by Clobazam and Ethosuximide since age 5. | Mild | Normal |
| 4 | – | – | 2 and 4 years : normal background, normal electroencephalogram during sleep and awakeness. | – | Moderate | Normal |
| 6 | 23 years | Focal epilepsy | 23 years : normal background, overload of diffuse slow waves. | Epilepsy controlled by Lamotrigine. | Severe | Moderate ventricular dilatation |
| 7 | <1 year | West syndrome | <1 year: Slow background, multifocal spikes when awake Slow anterior waves with spikes and polyspikes on bifrontal area, tonic seizures during sleep suggesting Lennox-Gastaut. | No information. | Severe | Normal |
| 8 | 4½ months | Focal epilepsy | 1 year: Slow background, spikes in the central areas, no activation in sleep. | Seizure free on Lamotrigine and Valproate. | Moderate | Normal |
| 9 | 9 months | West syndrome | 8 months : Absence of physiological organisation while awake, right posterior spikes, absence of physiological figures during sleep (hypsarrhythmia) 16 months : slow background with occipital spikes activated during sleep 18 months : slow background, biparietal spikes, spasms recorded 7 years : anterior spikes-waves alternating with slow sequences. | Inital treatment by Vigabatrin and corticotherapy. Spasms resistant to Ethosuximide, Lamotrigine, Valproate and ClobazamSpasms at last follow-up. | Profound | Aspecific white matter lesions |
| 10 | 7 months | Febrile seizures at 7 months and 3 years, myoclonic seizures at 8 years | 6 years : normal background, spikes-waves, left central slow waves when awake, activation during sleep without CSWS 9 years : normal background, right central-parietal spikes when awake with clinical seizures. | – | Severe | Thick corpus callosum |
| 12 | – | – | 8 years : normal background, right posterior slow waves when awake, normal sleep. | – | Severe | Normal |
| 13 | 5 months | West syndrome | 5 months : slow background, hypsarrhythmia, generalized spikes and poly-spikes with right predominance when awake, absence of physiological organization during sleep 10 months : slow background, right parietal-rolandic spikes, slow waves when awake, poor sleep organization 2 years : slow background, right rolandic spikes when awake, activated by sleep 2 years ½ : slow background, right parietal slow spikes when awake 9 years : normal background, posterior spikes-waves when awake. | Resistance to Vigabatrin, seizure free on Lamotrigine. | Moderate | Ventricular dilatation, corpus callosum hypoplasia |
| 15 | – | – | normal background, normal electroencephalogram. | – | Severe | Normal |
| 17 | 6 months | West syndrome | 6 months : slow background, hypsarrhythmia, absence of physiological organization 7 months : slow background, posterior spikes-waves during sleep 1 year : slow background, right posterior slow waves when awake. | Resistance to Vigabatrin, seizure free on Valproate. | Severe | Moderate myelinisation delay |
| 17 patients of our series | Patients from literature | Total of patients | Patients with the 4.5 Mb recurrent duplication | |
|---|---|---|---|---|
| Gender | 10F/7M | 14F/5M | 24F/12M | 15F/3M |
| Psychomotor delay | 16/17 | 9/16 | 25/33 (76%) | 10/15 (67%) |
| Language impairment | 17/17 | 17/19 | 34/36 (94%) | 17/17 (100%) |
| Intellectual disability | 16/17 | 18/18 | 34/35 (97%) | 16/17 (94%) |
| Behavior disorder | 8/17 | 5/14 | 13/31 (42%) | 6/16 (37%) |
| Attention deficit, hyperactivity disorder | 5/17 | 7/14 | 12/31 (39%) | 8/16 (50%) |
| Pervasive developmental disorder | 0/17 | 7/10 | 7/27 (26%) | 6/13 (46%) |
| Epilepsy | 9/17 | 8/18 | 17/35 (49%) | 6/17 (35%) |
| Electroencephalogram anomalies | 10/12 | 13/15 | 23/27 (85%) | 11/14 (78%) |
| Neurological examination anomalies | 7/17 | 8/16 | 15/33 (45%) | 7/16 (44%) |
| Macro/microcephaly | 6/17 | 5/16 | 11/33 (33%) | 5/15 (33%) |
| Brain MRI abnormalities | 6/14 | 6/11 | 12/24 (50%) | 4/11 (36%) |
| Sleep disorder | 8/17 | 2/3 | 10/20 (50%) | 5/7 (71%) |
| Early onset of puberty | 7/7F 0/8 M | 7/8F 1/3M | 14/15F (93%) 1/11M (9%) | 7/8F (88%) 1/1M |
| Overweight | 8/17 | 6/16 | 14/33 (42%) | 6/15 (40%) |
| Hypospadias | 2/8 | – | 2/8 (25%) | – |
| Anomalies of extremities | 5/14 | 11/13 | 16/27 (59%) | 12/16 (75%) |
- Patients with the 4.5 Mb recurrent duplication are displayed apart (last column).
The most typical features identified here and in previous works included developmental delay, ID, epilepsy, and early onset of puberty. Developmental delay was present in 25/33 cases (76%). Walking was acquired between 12 and 48 months (median walking age: 18 months). Most of the children (34/36 patients, 94%) had usually severe language impairment. Dysarthria or bucco-facial dyspraxia was noted in some cases. ID was the most prevalent feature, present in 34/35 cases (97%); it ranged from mild to severe and occurred regardless of sex and microduplication size. Nevertheless, Patient 9 (female), who had the largest duplication (8.9 Mb), presented encephalopathy with an inability to walk or speak. Aggressive behavior or anxiety was noted in 13/31 patients (42%). Attention deficit with or without hyperactivity disorder was noted in 12/31 patients (39%). Sleep disorders, especially night terrors, were reported in 10/20 patients (50%).
Epilepsy was present in 17/35 patients (49%). An electroencephalogram was performed in 27/35 patients with abnormalities in 23 (85%). Based on the classification of epileptic syndromes, we can describe four main groups of patients. In the first group, 4/27 patients (15%) presented with infantile spasms with hypsarhythmia consistent with West syndrome, defined by spasms during the first year, severe psychomotor delay, and hypsarhythmia. In this group, evolution was characterized by severe developmental delay, persistence of slow basal rhythm, and focal spikes activated during sleep. In the second group, 10/27 (37%) EEGs showed a normal background but with focal spikes and spikes-and-waves activated during sleep with frequent generalization. In some cases, continuous spikes-and-waves occurred during slow sleep. These patients had either no seizure or a wide range of paroxystic events such as night terrors or generalized tonic–clonic or myoclonic seizures. In the third group, 9/27 (33%) patients had focal seizures. For most of these patients, EEG during sleep was not available. Finally, in the fourth group, 4/27 (15%) patients did not experience seizures and had normal EEGs.
Abnormalities such as hypotonia, especially in West syndrome, were observed on neurological examination in 15/33 patients (45%). Brain magnetic resonance imaging (MRI) showed unspecific abnormalities such as cortical atrophy, ventricular dilatation, delayed myelinisation, or corpus callosum anomalies in 12/24 (50%) patients. There were no structural brain anomalies in patients with focal seizures. We found no correlation between brain anomalies and the severity of intellectual disability.
Endocrine abnormalities occurred frequently. Indeed, 14/15 (93%) females and one earliest reported male with the recurrent duplication had an early onset of puberty (mean age at menarche was 9 years). Two male patients in our series had hypospadias associated with micropenis and cryptorchidism, secondary to hypogonadism for one of them. Overweight starting at the age of four was observed in 14/33 (42%) patients. Accelerated growth in stature was observed during childhood, probably driven by the overweight and early onset of puberty. Mean final height in adulthood was 1m58 for males (−2.5 SD) (data were not available for females). A few patients also had immune disorders including hypothyroidism, nodular goiter, ulcerative colitis, Wegener's granulomatosis, uveitis, and thrombophilia.
Variable and non-specific anomalies of the extremities were observed in 16/27 (59%) patients such as camptodactyly, brachydactyly, clinodactyly of the fifth finger, tapering fingers, adductus thumb, hallux valgus, broad hallux, and syndactyly affecting the 2nd and 3rd toes.
Finally, although no facial feature can be easily recognized in this syndrome, there were some traits found relatively frequently in the subjects of this study, especially among females, including synophrys, bulbous nasal tip, short philtrum, and thin upper lip vermilion (Fig. 2).
DISCUSSION
In the present work, we present the clinical and molecular data of 17 new patients (10 females and 7males) harboring an overlapping duplication of the Xp11.23p11.22 interval, ranging in size from a minimum of 331 Kb to a maximum of 8.9 Mb. We also reviewed here the 19 patients previously reported by other authors.
In previous studies and in ours, a total of 18 patients with the recurrent 4.5 Mb Xp11.23p11.22 duplication have been reported (Table III). In these patients, clinical features included severe to mild ID (16/17), mostly severe speech delay (17/17), attention deficit/hyperactivity disorder (8/16), epilepsy (6/17), sleep disorder (5/7), early onset of puberty (8/9), and abnormal extremities (12/16). To date, a similar phenotype has been described in both males and females but additional observations in males are needed to confirm that these features are indeed independent of sex. The recurrent 4.5 Mb Xp11.23p11.22 duplication includes 47 genes of which 14 have already been associated with genetic disorders.
Among the 36 patients described in the literature and in our study, 11 patients from 8 different families have a small duplication defining minimal critical regions and candidate genes that could be responsible for specific clinical findings of the syndrome. Regarding neurological features, epilepsy, and EEG abnormalities including West syndrome, activation during sleep and focal seizures were found in 49% (17/35) and 85% (23/27) of the patients, respectively. The Xp11.23p11.22 duplicated regions in these patients allowed further refinement of the shortest region of overlap to a 24 Kb region (chrX:48,750,710–48,774,953) (Supplemental data file 2 - see supporting information online). For this interval, penetrance of the epileptic phenotype and of EEG abnormalities is about 60% (16/26) and 87% (21/24), respectively. The 24 Kb SRO segment contains four genes: TIMM17B, PQBP1, SLC35A2, and PIM2 (Supplemental file 1 - see supporting information online). Loss-of-function mutations in PQBP1 are associated with syndromic ID including microcephaly, short stature, spasticity, and occasionally heart or renal malformations [Kalscheuer et al., 2003; Lenski et al., 2004; Musante et al., 2010]. Protein encoded by PQBP1 is preferentially expressed in the brain [Waragai et al., 1999]. In cellular models, overexpression of the PQBP1 protein decreases cell growth and increases sensitivity to stress. Furthermore, transgenic mice overexpressing PQBP1 presents progressive motor neuron impairment. More precisely, the pathological examination of these transgenic mice revealed a loss of Purkinje and granular cells in the cerebellum and a loss of spinal motor neurons, supporting the role of PQBP1 in neuronal function [Okuda et al., 2003]. Recently, de novo mutations have been identified in SLC35A2 gene in six patients with congenital disorder of glycosylation type IIm, including four females and two males, with five having seizures [Kodera et al., 2013]. EEG exhibited hypsarhythmia in four patients and multifocal spike and slow wave complex in the last one. The two other genes (TIMM17B and PIM2) of this region have not been shown to be involved in any developmental disorder so far. We found no correlation between the size of the duplication and the severity of epilepsy; for e.g., Patients 13 and 17 presented West syndrome despite small duplications extending from 331 to 454 Kb. It is also worth noting that the mother of Patient 3 reported by Giorda et al., harboring a small duplication including PQBP1 and SLC35A2, had episodes of cyanosis and blank staring suggesting possible absences without ID. Finally, the duplication detected in Patient 17 included neither PQBP1 nor SLC35A2. However, the centromeric breakpoint was located approximately 102 Kb away from these genes. The expression level of the PQBP1 gene might be affected by an alteration of an upstream regulatory element of the gene (i.e., a position effect) [Benko et al., 2011].
ID is a main feature even though one of our patients (Patient 1) had a borderline evaluation (IQ of 75) and another (Patient 2) had an initially normal evaluation followed by moderate cognitive decline during childhood. FTSJ1 and SHROOM4 genes are particularly interesting as concerns developmental delay (Fig. 1). One of the smallest region of overlap with respect to the ID is defined by the 331 Kb duplication of Patient 17. The duplication encompassed 11 RefSeq genes including FTSJ1, a gene expressed in brain tissue. Loss-of-function mutations in FTSJ1 have been reported in five patients with ID and aggressive behavior but no genetic functional study has been performed [Freude et al., 2004; Ramser et al., 2004; Takano et al., 2008]. As for SHROOM4, which is duplicated only in patients with the recurrent or a large duplication, loss-of-function mutations have been identified in patients from three families and a missens mutation in one large family with moderate to severe ID, speech delay, epilepsy, and abnormal extremities [Hagens et al., 2006; Honda et al., 2010]. However, other studies are needed to replicate those results [Piton et al., 2013]. It is also worth noting that the 410 Kb telomeric region near the M-REP contains seven other genes involved in ID or brain function: KCND1 (potassium voltage-gated channel, Shal-related subfamily, member 1) codes a voltage-gated potassium channel expressed in the brain [Pak et al., 1991]; GRIPAP1 (GRIP1 associated protein 1) has a role in dendritogenesis, synaptic vesicle release, and AMPA receptor exocytosis [Ye et al., 2007]; PRAF2 (PRA1 domain (colonic epithelium-enriched) codes a protein localized on synaptic vesicle membranes [Fo et al., 2006; Koomoa et al., 2008]; PLP2 (proteolipid protein 2 (colonic epithelium-enriched) codes a membrane protein expressed in the brain and promoter mutations in this gene have been associated with ID [Zhang et al., 2007]; SYP (Synaptophysin) codes a protein of the synaptic vesicle membrane and mutations have been associated with ID and epilepsy [Tarpey et al., 2009]; CCDC22 (coiled-coil domain containing 22) has been found to be mutated in patients with ID [Voineagu et al., 2012]; and PPP1R3F (Protein phosphatase 1, regulatory (inhibitor) subunit 3 F) has been found to be mutated in patients with pervasive development disorder. Nonetheless, among these genes, FTSJ1 and SHROOM4 appear to be the best candidates for developmental delay.
Regarding early onset of puberty, the main candidate gene is BMP15 (bone morphogenetic protein 15) as previously reported in the literature [Giorda et al., 2009]. BMP15 codes an oocyte-specific growth and differentiation factor that stimulates synthesis and secretion of FISH by the pituitary gland [Otsuka and Shimasaki, 2002; Di Pasquale et al., 2004]. Loss-of-function mutations have been associated with gonadal dysgenesis and premature ovarian failure [Rossetti et al., 2009]. Overexpression of BMP15 has been shown to accelerate follicular growth in mouse models [McMahon et al., 2008].
In males, structural X disomy always results in functional X disomy and subsequently in an abnormal phenotype. In most cases, the anomaly is inherited from the mother who is a healthy carrier. Indeed, female carriers show severely skewed X-inactivation of the rearranged X chromosome and thus are asymptomatic. In the present situation, females carrying a recurrent Xp11.23p11.22 or a larger microduplication are affected. Interestingly, there are many more affected females than males in the literature and in our study; in all 24 females versus 12 males. In our series, symptomatic carriers (n = 7) did not exhibit more skewed XCI patterns than asymptomatic carriers (n = 3), with the exception of Patient 1 who had an IQ of 75 and a severely skewed XCI profile. Thus, the extent of XCI skewing can not be used for clinical practice to predict the phenotypic severity of this syndrome. Moreover, the mothers carrying a smaller microduplication than the recurrent one were asymptomatic and showed variable X-inactivation patterns (i.e., skewed XCI for the mothers of Patients 14–16 and 17 and random XCI for the mothers of Patients 11– 12, and Case 3 from Giorda et al.). Thus, a small Xp11.23p11.22 microduplication does not appear to be sufficient to induce ID in females regardless of X-inactivation pattern. Cognitive impairment was only observed in females with a recurrent or larger Xp11.23p11.22 microduplication.
Several studies demonstrated that genes escaping X chromosome inactivation in humans are distributed in clusters and located on the short arm of the X chromosome, especially in the Xp11 region [Disteche, 1999; Tsuchiya et al., 2004; Carrel and Willard, 2005]. Moreover, Zhang et al. showed that there is an excess of X-inactivation escaping genes associated with mental impairment, including FTSJ1, PQBP1, and GRIPAP1 [Zhang et al., 2013]. Thus, it can be hypothesized that some duplicated genes escape from X-inactivation, leading to functional disomy of X-linked genes and subsequently clinical manifestations in females.
Among the 36 patients who have been reported, 15 females and three males were carriers of the 4.5 Mb recurrent duplication. This rearrangement is mediated by NAHR between the paralogous distal (D-REP) and proximal (P-REP) repeat sequences. Non-recurrent Xp11.23p11.22 duplications were detected in the remaining 18 patients. The DNA-replication-based fork stalling and template switching (FoSTeS) as well as microhomology-mediated break-induced replication (MMBIR) or non-homologous end joining (NHEJ) mechanisms may underlie these rearrangements. The presence of low copy repeats in the region may generate an unstable DNA structure that can induce DNA strand lesions and facilitate a FoSTeS mechanism [Lee et al., 2007; Zhang et al., 2009]. The recurrent Xp11.23p11.22 microduplications are usually de novo in females. Paternal origin of these rearrangements was demonstrated in all these cases [Giorda et al., 2009]. Thus, NAHR probably occurs between sister-chromatids during paternal meiosis [Gu et al., 2008].
CONCLUSION
The human Xp11.23p11.22 region is susceptible to recurrent chromosome rearrangements leading to 4.5 Mb microduplications mediated by NAHR or variably-sized non-recurrent duplications caused by a FoSTeS mechanism. In the present study, to further delineate phenotype–genotype correlations, we described 17 new patients, 10 females, and 7 males. These microduplications, both in females and males, are associated with ID, speech disorder, early onset of puberty, and epilepsy such as West syndrome and focal seizures with occasional activation during sleep. The extent of XCI skewing does not appear to predict the phenotypic severity of this syndrome. We propose several candidate genes for specific phenotypes, such as FTSJ1 and SHROOM4 for ID and PQBP1 and SLC35A2 for epilepsy. We reinforce the implication of BMP15 for early onset of puberty. A transcriptomic approach could evaluate how Xp11.23p11.22 duplications affect the expression of these genes. Furthermore, additional individuals with this chromosomal rearrangement will be helpful in gaining a better understanding of the pathogenesis of this condition.
ACKNOWLEDGMENTS
We thank our colleagues for referring patients, and the patients and their families for participating in this study.




