Seizures and EEG features in 74 patients with genetic-dysmorphic syndromes
Abstract
Epilepsy is one of the most common findings in chromosome aberrations. Types of seizures and severity may significantly vary both between different conditions and within the same aberration. Hitherto specific seizures and EEG patterns are identified for only few syndromes. We studied 74 patients with defined genetic-dysmorphic syndromes with and without epilepsy in order to assess clinical and electroencephalographic features, to compare our observation with already described electro-clinical phenotypes, and to identify putative electroencephalographic and/or seizure characteristics useful to address the diagnosis. In our population, 10 patients had chromosomal disorders, 19 microdeletion or microduplication syndromes, and 32 monogenic syndromes. In the remaining 13, syndrome diagnosis was assessed on clinical grounds. Our study confirmed the high incidence of epilepsy in genetic-dysmorphic syndromes. Moreover, febrile seizures and neonatal seizures had a higher incidence compared to general population. In addition, more than one third of epileptic patients had drug-resistant epilepsy. EEG study revealed poor background organization in 42 patients, an excess of diffuse rhythmic activities in beta, alpha or theta frequency bands in 34, and epileptiform patterns in 36. EEG was completely normal only in 20 patients. No specific electro-clinical pattern was identified, except for inv-dup15, Angelman, and Rett syndromes. Nevertheless some specific conditions are described in detail, because of notable differences from what previously reported. Regarding the diagnostic role of EEG, we found that—even without any epileptiform pattern—the generation of excessive rhythmic activities in different frequency bandwidths might support the diagnosis of a genetic syndrome. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Genetic-dysmorphic syndromes are characterized by the association of major and minor malformations (dysmorphisms). They often include variable degrees of psychomotor delay and other various neurological symptoms, among which epilepsy is one of the most common [Schinzel and Niedrist, 2001; Bahi-Buisson et al., 2005; Battaglia and Guerrini, 2005; Kumada et al., 2005; Parmeggiani et al., 2005; Yamanouchi et al., 2005].
A specific electro-clinical phenotype has been identified only for a limited group of syndromes, e.g., Angelman [Pelc et al., 2008], Rett [Glaze et al., 2010; Nissenkorn et al., 2010; Cardoza et al., 2011], Wolf-Hirschhorn [Battaglia et al., 2009], and inv dup(15) syndromes [Battaglia, 2008]. In the majority of genetic syndromes, the seizure types and the EEG patterns vary considerably and consequently fail to characterize specific conditions. It is also generally agreed that EEG can provide only a negligible contribution to the diagnosis, particularly in patients without epileptic seizures [Shevell et al., 2003; McDonald et al., 2006].
The aim of the present study was to recognize the epileptic phenotype and the EEG characteristics of patients with defined genetic syndromes in order to identify any novel diagnostic indicators and to verify the presence of EEG-clinical patterns already acknowledged as being specific for certain syndromes.
MATERIALS AND METHODS
We included 74 (34 females) pediatric and young adult patients (age range 1–31 years at the moment of our last observation) referred to the Developmental Neurology Unit between 2007 and 2011 because of suspected genetic syndromes. We selected the patients provided that they had a positive diagnosis of a genetic syndrome and completed at least one EEG including awake and spontaneous afternoon nap recording.
The diagnosis of the genetic syndromes relied on cytogenetic tests (standard karyotype), cytogenetic-molecular studies (FISH for specific regions and for the subtelomeric regions), or molecular investigations (Fragile X, methylation test for Angelman/Prader-Willi syndromes, array-CGH, molecular studies on specific genes). When laboratory data were unable to clarify the genetic determinants, we based the diagnosis on clinical criteria acknowledged by international literature [Scott et al., 1971; Ruiz-Maldonado et al., 1992; Somer, 1993; Evers et al., 1995; Opitz et al., 1998; De Falco et al., 2003; Brancati et al., 2010].
Clinical assessment included a general examination aimed to detect and classify dysmorphisms, anthropometric data, and objective neurological findings. Patients' cognitive levels were assessed using the Wechsler scales (WPPSI; WISC-R or WISC-III; WAIS) depending on their age or using a psychomotor development scale (Griffiths' Mental Development Scale) in younger patients or in those who could not be administered the Wechsler scales.
Patients were grouped both on the basis of the type of genetic defect or as clinical diagnosis (four groups: chromosomal, microdeletion/microduplication, monogenic, clinical) and according to the epileptic phenotype (three groups: epileptic encephalopathy, focal epilepsy, generalized epilepsy). Epileptic encephalopathies were defined in agreement with the International League against Epilepsy (ILAE) criteria [Engel, 2006] in the presence of frequent seizures and EEG discharges associated with the worsening of cognitive function. Epilepsies were defined as focal in the presence of electro-clinical features suggesting a focal or multi-focal onset, while were defined as generalized in the presence of generalized tonic-clonic seizures (without any clinical sign of focal onset) associated with diffuse epileptiform anomalies. Severity of epilepsy was also reported. The classification of seizure and epilepsies was made according to the International Classification of Seizures [ILAE, 1981; Fisher et al., 2005; Berg et al., 2010] and Epileptic Syndromes [ILAE, 1989; Fisher et al., 2005; Berg et al., 2010] proposed by the ILAE.
The EEG was recorded using Ag/AgCl electrodes placed according to the International 10–20 system; polygraphic signals included electro-oculogram (ECG), pneumogram, submental EMG, and additional surface EMG (including at least the deltoid muscles). Moreover, time-locked video monitoring was associated when appropriate. Signals were acquired by a computerized system (Micromed SpA, Mogliano Veneto, Italy).
We evaluated the EEG signal's organization while awake and asleep and the morphology, localization, occurrence, and incidence of any paroxysmal and/or epileptiform anomalies, according to the International Guidelines of Neurophysiology [Nuwer et al., 1999; Flink et al., 2002].
Magnetic resonance imaging (MRI) was performed in all patients, with sagittal, transverse, coronal, and coronal fluid-attenuated inversion recovery (FLAIR) sequences (0.5 T and/or 1.5 T). Brain MRI was classified as: (1) normal; (2) with signs indicating pre-perinatal stress; (3) with signs of maldevelopment, (including cortical focal migration and gyration anomalies, or dysembriogenetic cortical neoplasms);and (4) with abnormalities exceeding the cerebral cortex (e.g., corpus callosum hypoplasia, cerebellar hypoplasia or dysplasia, “molar tooth” sign, Chiari type I malformations, arachnoid cysts, hydrocephalus).
RESULTS
Sixty-one (82.4%) of the 74 patients, had their diagnoses confirmed genetically by specific laboratory tests: 10 (13.5%) had a chromosomal disorder, 19 (25.7%) had microdeletion or microduplication syndromes, and 32 (43.2%) had a monogenic condition. The remaining 13 (17.6%) were classified on clinical grounds (Table I).
| Chromosomal disorders | 10 (13.5%) |
| 2 Klinefelter | |
| 2 inv dup(15) | |
| 1 mos47, XY + r (14)/46, XY (mosaic ring 14) | |
| 1 mos47, XY + 13/46, XY (mosaic Patau) | |
| 1 Pallister-Killian | |
| 1 del(13)(q32–q32) | |
| 1 supernumerary marker chromosome from chromosome 13 | |
| 1 dup(13)(q32–q34) | |
| Microdeletion/microduplication syndromes | 19 (25.7%) |
| 4 Angelman | |
| 2 Smith-Magenis | |
| 2 del22q13 (Phelan-McDermid) | |
| 1 Wolf-Hirschhorn | |
| 1 Rubinstein-Taybi | |
| 1 Prader-Willi | |
| 1 PEHO/dup(8)(q11.23) | |
| 1 del22q11.2 (DiGeorge) | |
| 1 rec(X)dup(Xq)inv(X)(p22.2q26)mat | |
| 1 del(22)(q12.1–12.2) + dup(22)(q13.31–13.33) | |
| 1 dup(X)(q28) | |
| 1 del(14)(qter) | |
| 1 del(12)(qter) + dup(10)(p15.3–p13) | |
| 1 “cri du chat” (5p-) | |
| Monogenic conditions | 32 (43.2%) |
| 6 Rett | |
| 5 Noonan | |
| 3 CHARGE | |
| 2 Costello | |
| 2 Fragile X | |
| 2 Sotos | |
| 2 Mowat-Wilson | |
| 1 Cowden | |
| 1 Cohen | |
| 1 Coffin-Lowry | |
| 1 Joubert | |
| 1 COACH (JS-H) | |
| 1 ATRX | |
| 1 Baller-Gerold | |
| 1 Waardenburg | |
| 1 Usher | |
| 1 Alstrom | |
| Clinical diagnoses | 13 (17.6%) |
| 4 Joubert | |
| 2 Varadi-Papp (JS-OFD) | |
| 2 Hypomelanosis of Ito | |
| 1 COACH (JS-H) | |
| 1 Weaver | |
| 1 Scott craniodigital | |
| 1 Opitz | |
| 1 Aplasia cutis congenita |
In 11 of the 13 patients with undetectable biogenetic markers, the array-CGH analysis ruled out the presence of microdeletions or microduplications. In the other two, the diagnosis (Hypomelanosis of Ito in one and Aplasia cutis congenita in the other) was established based on acknowledged diagnostic criteria [Ruiz-Maldonado et al., 1992; Evers et al., 1995].
Table II shows the main clinical findings including family history, clinical and neurological findings, and neuroradiological characteristics.
| Family history | No. | % |
|---|---|---|
| Family history of epilepsy | 9 | 12.2 |
| Family history of febrile seizures | 3 | 4.1 |
| General examination | ||
| Changes in stature and weight: | 46 | 62.2 |
| lower or higher than normal stature/obesity | 21 | 28.4 |
| microcephaly | 14 | 18.9 |
| macrocephaly | 11 | 14.9 |
| Major malformations | 8 | 10.8 |
| Facial dysmorphisms | 40 | 54.1 |
| Neurological findings | ||
| Psychomotor delay or intellectual disability | 63 | 85.1 |
| Pyramidal signs | 16 | 21.6 |
| Extrapyramidal signs | 27 | 36.5 |
| Ataxia | 17 | 23.0 |
| Altered ocular motility (oculomotor apraxia, oculomotor impairment) | 9 | 12.2 |
| Seizures/Epilepsy | ||
| Febrile seizures | 6 | 8.1 |
| Neonatal seizures | 2 | 2.7 |
| Epilepsy | 27 | 36.5 |
| Age at onset of epilepsy | ||
| 0 to 12 months old | 8 | 10.8 |
| 1 to 6 years old | 13 | 17.5 |
| 6 to 15 years old | 3 | 4.1 |
| over 15 years old | 3 | 4.1 |
| Neuroradiological findings | ||
| Normal brain MRI | 30 | 40.5 |
| Brain MRI with anomalies | 44 | 59.5 |
| Sequelae of fetal-connatal stress | 4 | 5.4 |
| Cortical dysplasias or dysembriogenetic neoplasms | 4 | 5.4 |
| Other anomalies “not involving the cerebral cortex” | 36 | 48.7 |
Most of the patients (85.1%) had psychomotor delay or intellectual disability, often associated with other neurological signs.
MRI was normal or with changes that did not involve the cerebral cortex in most cases (89.2%). Only a minority of the patients (8/74; 10.8%) had cortical or subcortical changes known to be a potential cause of epileptic seizures (or a predisposing factor), and only one of these latter had epilepsy.
Epilepsy and EEG Findings
Twenty-seven patients (36.5%) had epilepsy (present or previous); diagnoses were focal epilepsy in 16 cases, epileptic encephalopathy in six, epilepsy with generalized tonic-clonic seizures in five. Six infants had febrile seizures (8.1%) and two had neonatal convulsions (2.7%).
Classification and severity of epilepsy and the main characteristics of EEG findings are reported in Table III.
| Genetics | Epileptic encephalopathy | Focal epilepsy | Generalized epilepsy |
|---|---|---|---|
| Chromosomal (total n = 5) | |||
| Klinefelter | 1 | ||
| supernumerary marker ch 13 | 1 | ||
| dup(13)(q32–q34) | 1 | ||
| Inv dup(15) (n = 2) | 2 | ||
| Microdeletion/microduplication (total n = 7) | |||
| Angelman (n = 3) | 3 | ||
| del22q13 (Phelan-McDermid) | 1 | ||
| PEHO/dup(8)(q11.23) | 1 | ||
| del(12)(qter) + dup(10)(p15.3–p13) | 1 | ||
| del(22)(q12.1–12.2) + dup(22)(q13.31–q13.33) | 1 | ||
| Monogenic (total n = 11) | |||
| Rett (n = 4) | 1 | 3 | |
| Costello (n = 2) | 2 | ||
| Mowat-Wilson (n = 2) | 1 | 1 | |
| Noonan | 1 | ||
| Waardenburg | 1 | ||
| CHARGE | 1 | ||
| Clinically defined (total n = 4) | |||
| COACH (JS-H) | 1 | ||
| Opitz | 1 | ||
| Scott | 1 | ||
| Hypomelanosis of Ito | 1 | ||
| Seizure typesa | Myoclonic (n = 3); TC (n = 3); atypical absences (n = 1); polymorphic (n = 1); spasms (n = 1) | Focal (n = 14); focal with secondary generalization (n = 2) | TC (n = 5); myoclonic (n = 1); absences (n = 1) |
| AED resistance | 5/6 | 6/16 | 0/5 |
| EEG epileptic activities | 83.3% | 81.2% | 80.0% |
| EEG rhythms excess | 66.7% | 62.5% | 80.0% |
| Total | n = 6 | n = 16 | n = 5 |
- a Some patients had more than one type of seizure.
Severe epilepsy with drug resistant seizures occurred in 11/27 (40.7%) patients; they were affected by Angelman (3/4) and Rett syndrome (4/6); one of the two patients affected by del22q13 (Phelan-McDermid) had severe epileptic encephalopathy (Lennox-Gastaut syndrome); other three patients developed drug resistant focal epilepsy (Costello, Waardenburg, Scott).
In the remaining 16 cases (59.3%), seizures were controlled by anti-epileptic drugs.
At that time of our examination, only 20 patients (27.0%) had a completely normal EEG both awake and during sleep. Among them, none had active seizures but four had seizures/epilepsy in their clinical history: febrile seizures in two (Klinefelter syndrome and ATRX syndrome), West syndrome in one (PEHO/dup8q11.23), and few focal seizures responsive to Valproate in one (supernumerary marker chromosome from chromosome 13).
Thirty-six patients (48.6%) had epileptiform anomalies on their EEG, but more than one third of them (14/36) had no epileptic event in their history. Abnormalities were certainly more represented in patients with active epilepsy (epileptic encephalopathy, focal epilepsy), but also in a significant portion of patients without any history of epilepsy (14/47, 29.8%).
Nine out of these 36 patients (12.2%) had a normal awake and sleep EEG organization. Their epileptic abnormalities were focal in six, multifocal in one, diffuse in one, and multifocal with features fitting those of idiopathic focal childhood epilepsy in one (mosaic Patau). The incidence of these epileptiform anomalies was rare in four cases, moderately frequent in another four, and very frequent in one. Among these patients, two had focal epilepsy (Klinefelter, Waardenburg), two had febrile seizures (Noonan, Cohen), and five did not have any history of seizures.
The remaining 27 patients (36.5%) with epileptiform anomalies showed also a poor awake and sleep EEG organization. But another important characteristic in these patients was the presence of an excess of diffuse rhythmic activity, involving variable frequencies in beta, alpha, and theta band. Epileptic abnormalities were focal in six, diffuse in two, and multifocal in 16. The remaining three patients (del22q13; Varadi-Papp; Aplasia cutis congenita) had an EEG epileptiform pattern consistent with those of idiopathic focal childhood epilepsy; none of these three patients had epileptic seizures. The epileptic anomalies occurred only rarely in eight cases, more frequently in 10, and very often in nine cases.
Among these 27 cases, five had epileptic encephalopathy, 12 had focal epilepsy, three epilepsy with generalized tonic-clonic seizures, and seven did not have any history of seizures.
Three patients (4.1%) (COACH, Joubert, Wolf-Hirschhorn) showed only a poor sleep organization without epileptic abnormalities, in spite of the fact that one had still present focal seizures (i.e., COACH).
Considering all groups, the main EEG characteristic was a poor awake and sleep EEG organization, observed in the majority of patients (42; 56.7%). Moreover most of them (34; 45.9%) revealed an excessive diffuse rhythmic activity, involving frequencies in beta, alpha, and theta band. Particularly, excesses of rhythmic activity in beta band specially occurred during drowsiness and light sleep, while high-amplitude alpha–theta rhythms (namely on fronto-central regions) come into view during slow-wave sleep (Figs. 1 and 2).
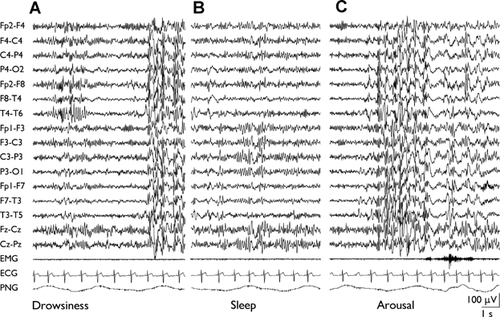
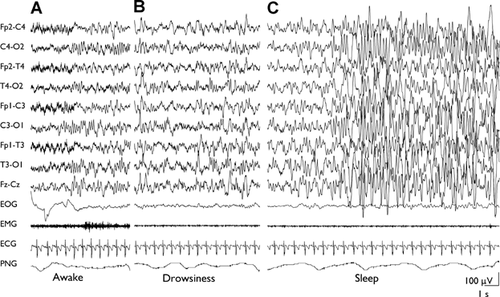
The finding of these abnormal EEG rhythmic activity was especially evident in 27 patients (i.e., two inv dup(15), four Angelman, two Smith-Magenis, two del22q13, one del22q12.1–12.2 + dup22q13.31–13.33, one dup13q32q34, one Cri du chat, three of six Rett, two of five Noonan, two Fragile X, one of two Mowat-Wilson, two Costello, one of five Joubert, one Coffin-Lowry, one of two Hypomelanosis of Ito, one Scott craniodigital) and appeared to be a characteristic EEG trait in our population.
DISCUSSION
Our observation of 74 patients with genetic-dysmorphic syndromes confirms a rather high incidence of epileptic seizures (36.5%), in line with that previously reported in other series of patients with chromosomal abnormalities [Schinzel and Niedrist, 2001; Singh et al., 2002; Bahi-Buisson et al., 2005; Parmeggiani et al., 2005] and point out the rather heterogeneous phenotypic presentation of epilepsy and epileptiform EEG features. A rather common excess of rhythmic EEG activities in more than a half of our patients may be considered as a useful marker of these disorders, even in the absence of a specific disease-linked seizure or EEG pattern.
Epilepsy and Seizures
Among our 27 patients with epilepsies, seizures were drug-resistant in more than one third, a percentage that is in line with those reported for other symptomatic epilepsies [Picot et al., 2008]. In our series, we also found a rather high incidence of febrile (8.1%) and neonatal seizures (2.7%), certainly higher with respect to that of general population (2–5% and 0.1–0.5%) [Shinnar and Glauser, 2002; Tharp, 2002], suggesting that genetic-dysmorphic syndromes can act as a predisposing factor toward seizures and sustain mild and transient epileptic phenotypes. The EEG and clinical presentation suggested that, in addition to epileptic encephalopathies occurring in already known genetic syndromes, focal or multi-focal seizures characterized most patients, but the relationship between seizure types and specific syndromes was not sufficiently consistent to propose definite electro-clinical patterns.
Syndromes Already Known to be Associated With Severe Epilepsies
In agreement with the literature data, epilepsy was definitely severe in some specific syndromes already known to couple with epileptic encephalopathies, as in Angelman and Rett syndromes [Pelc et al., 2008; Nissenkorn et al., 2010; Williams et al., 2010; Cardoza et al., 2011] and, to a less extent, in patients with inv dup(15) [Battaglia, 2008] and Wolf-Hirschhorn syndrome [Battaglia et al., 2009]. In these disorders, our observation well fits the typical described presentations in terms of age of onset, seizure types, and EEG patterns.
Seizures in Syndromes Usually not-Associated With Severe Epilepsies
In our case series, recurrent seizures occurred in genetic-dysmorphic pictures that are unexpected to present with severe epilepsy, including Phelan-McDermid, Noonan, Costello, Joubert-related syndromes, Waardenburg, Scott craniodigital, and Opitz.
Among patients with disorders resulting from microdeletions, one patient with the 22q13 microdeletion (Phelan-McDermid syndrome) had severe drug-resistant epilepsy with everyday polymorphic seizures from the age of four years. He was treated with different associations of anti-epileptic drugs, underwent to surgical callosotomy, and subsequent vagal nerve stimulation (VNS). This presentation can be considered unusual and has not been previously described; indeed, only mild and infrequent (25–30%) epilepsies have been previously reported [Cusmano-Ozog et al., 2007; Phelan, 2008; Dhar et al., 2010].
Our series included seven cases with monogenic disorders of Ras/MAPK pathway, five classified as Noonan, and two as Costello syndrome. Epilepsy has been rarely reported in both syndromes [Duenas et al., 1973; Sharland et al., 1992; Colli et al., 2001; Romano et al., 2010], while recurrent seizures were observed in only one patient associating cortical dysplasia [Saito et al., 1997]. In line with previous observations among our five patients with Noonan syndrome (all with PTPN11 gene mutations), only two had seizures which presented in one as isolated febrile seizure (and EEG anomalies) and in the other one with late focal seizures well responding to anti-epileptic treatment. In both, brain MRI did not reveal dysplastic changes in the cerebral cortex. Severe epilepsy characterized on the contrary both our patients with Costello syndrome. Seizure are considered a possible but not prominent symptom in this syndrome [Delrue et al., 2003; Kawame et al., 2003], while both our patients had a prominent epileptic presentation with early infantile onset in one and with a juvenile onset in the other one, who then developed drug-resistant focal seizures.
All but one of our nine patients with Joubert or Joubert-related syndromes, including five with typical Joubert, two with COACH (or Joubert syndrome with hepatic defect, JS-H), and two with Varadi-Papp (or Joubert syndrome with orofaciodigital defects, JS-OFD) syndrome had no epilepsy, in line with literature data indicating that epilepsy seldom occurs in these syndrome [Brancati et al., 2010; Parisi, 2009]. However, one patient with COACH syndrome (JS-H) had recurrent focal seizures, a phenotype that was not previously reported in the literature.
Prominent and severe epilepsies characterized individual patients with other rare genetic-dysmorphic syndromes in which significant epilepsy has not been previously reported. One patient had Waardenburg syndrome and presented with early onset drug-resistant focal seizures differing from the only occasional and unimportant seizures reported in the literature [Sanyas et al., 1982; Cantani et al., 1989]. Recurrent and drug-resistant focal seizures were also present in a patient with Scott craniodigital syndrome [Milani et al., 2007] and in one with Opitz syndrome, although the previously described patients with both these syndromes only occasionally had seizures [Lorenz et al., 1990; De Falco et al., 2003].
On the whole, significant epilepsy often poorly responsive to anti-epileptic drugs appears to characterize our sample of patients, suggesting that epileptic disorder can associate and represent a significant clinical problem in a variety of genetic syndromes not included in those typically leading to epileptic encephalopathies.
The EEG recording is rarely considered as an important tool in the diagnostic evaluation of children with non-progressive global developmental delay [Shevell et al., 2003]. The Report from the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society recommended that “an EEG can be obtained when a child with global developmental delay has a history or examination features suggesting the presence of epilepsy or a specific epileptic syndrome”. This recommendation stemmed from studies by Shevell et al., [2001], and Battaglia et al., [1999] involving 200 (80 and 120, respectively) children with global developmental delay who underwent diagnostic evaluation. An analysis of these studies found that EEG did not help to ascertain the etiology of their encephalopathy and that 8.3% of these children had epileptic syndromes (in which case an abnormal EEG was to be expected). These studies have also been considered an important milestone in subsequent reviews [van Karnebeek et al., 2005]. On the other hand, in past years, particular EEG features have been recognized in various chromosomal genetic encephalopathies, e.g., Angelman and Rett syndromes [Viani et al., 1995; Laan et al., 1998; Minassian et al., 1998; Glaze, 2002; Bahi-Buisson et al., 2008], in which the EEG features have remarkable predictive value in the diagnosing work-up.
In recent years, much effort has gone into molecular genetic studies with the purpose of discovering etiological basis of these diseases, while EEG, giving information about the resulting dysfunction, has not been adequately described in the series reported in the literature [Shevell et al., 2003].
In our sample, the reappraisal of awake and asleep EEG recordings enabled us to add further considerations to the already known contributions on several specific conditions.
In many patients, even in those not suffering from epilepsy, the EEG revealed characteristics that, in our opinion, may significantly contribute to the diagnosis. Among these characteristics, the presence of excessive rhythmic activities in different frequency bandwidths was the most peculiar finding, shared by several genetic syndromes [Goldman et al., 2006]. Actually, high voltage EEG fast rhythms are known to be a typical finding in lissencephaly [Gastaut et al., 1987], but have been described as a non strictly specific finding also in other overt brain malformations [Quirk et al., 1993; Raymond and Fish, 1996], thus suggesting that the pattern of increased rhythmic alpha–beta activity may represent the EEG pattern reflecting widespread cortical and subcortical “malformative” finding, not detectable on neuroradiological images or “dysfunctional” circuitries due to microscopic disruption of the cortical mantle.
These characteristics, together with the finding of a high percentage of patients with no epileptic seizures but with epileptiform anomalies on their EEG, even not-specific, can be useful in orienting the diagnosis in patients with suspected genetic-dysmorphic syndromes or undetermined encephalopathies. Further systematic studies on the electro-clinical patterns in patients with genetic syndromes on large samples may help to reach a better characterization of the various phenotypes.
ACKNOWLEDGMENTS
We thank all the neurophysiology technicians at the Carlo Besta Neurological Institute in Milan.