Intragenic rearrangements in X-linked intellectual deficiency: Results of a-CGH in a series of 54 patients and identification of TRPC5 and KLHL15 as potential XLID genes
Abstract
High-resolution array comparative genomic hybridization (a-CGH) enables the detection of intragenic rearrangements, such as single exon deletion or duplication. This approach can lead to the identification of new disease genes. We report on the analysis of 54 male patients presenting with intellectual deficiency (ID) and a family history suggesting X-linked (XL) inheritance or maternal skewed X-chromosome inactivation (XCI), using a home-made X-chromosome-specific microarray covering the whole human X-chromosome at high resolution. The majority of patients had whole genome array-CGH prior to the selection and we did not include large rearrangements such as MECP2 and FMR1 duplications. We identified four rearrangements considered as causative or potentially pathogenic, corresponding to a detection rate of 8%. Two CNVs affected known XLID genes and were therefore considered as causative (IL1RAPL1 and OPHN1 intragenic deletions). Two new CNVs were considered as potentially pathogenic as they affected interesting candidates for ID. The first CNV is a deletion of the first exon of the TRPC5 gene, encoding a cation channel implicated in dendrite growth and patterning, in a child presenting with ID and an autism spectrum disorder (ASD). The second CNV is a partial deletion of KLHL15, in a patient with severe ID, epilepsy, and anomalies of cortical development. In both cases, in spite of strong arguments for clinical relevance, we were not able at this stage to confirm pathogenicity of the mutations, and the causality of the variants identified in XLID remains to be confirmed. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
The sex ratio of ID patients is biased toward males, underlining an important role played by X-linked genes. X-linked mutations could be responsible for up to 12% of male ID cases having a genetic origin [Ropers and Hamel, 2005; Alesi et al., 2012]. These XLID cases are most often recessively inherited from unaffected carrier females to affected male offspring.
Currently, approximately 100 XLID genes have been identified [Chiurazzi et al., 2008; Tarpey et al., 2009; Ropers, 2010; Lubs et al., 2012; Piton et al., 2013]. Nonetheless, numerous XLID pedigrees still await gene identification. More than 300 X-linked genes encode proteins expressed in the brain, suggesting that many additional causes of ID remain to be identified [Gecz et al., 2009]. The identification of these genes should allow to better understand the mechanisms underlying cognition or brain development and, possibly, to improve the treatment of the neurological signs together with a better genetic counseling for affected families.
During the last 10 years, comparative genomic hybridization (CGH) techniques have been used to search for rearrangements on the X-chromosome in individuals suffering from XLID in order to identify new genetic causes [Froyen et al., 2007; Lugtenberg et al., 2007; Madrigal et al., 2007; Isrie et al., 2012]. The improvement of this technique through the development of high-resolution arrays has allowed the identification of small rearrangements, some of them containing a single gene (or portion of a gene).
Here, we report on the analysis of 54 male patients presenting with ID using a homemade X-chromosome-specific genomic microarray covering the whole human X-chromosome at high resolution. Several rearrangements were identified and we discuss their putative involvement in the phenotype of the patients following a decision scheme that we have adopted after a review of similar studies [Isrie et al., 2012].
MATERIALS AND METHODS
Patients
These 54 male patients were selected from a series of patients with intellectual deficiency (ID) from our Centre of Reference for Developmental Abnormalities. Twenty probands originated from families with suspected X-linked pattern of inheritance (affected individuals in more than one sibship); 30 probands originated from affected brothers or half-brothers pairs; and four sporadic male cases had highly skewed X-chromosome inactivation (XCI) in their mother (XCI ratio >80:20). The majority of the patients had 44K or 180K full genome array-CGH prior to the selection. Two MECP2 duplications and one FMR1 duplication identified in this first step were excluded from further analysis. One of the patients was screened using array-CGH in order to confirm a deletion suspected following PCR failure during routine mutational screening of the OPHN1 gene. Written parental informed consent was obtained in all cases.
Comparative Genomic Hybridization
The Roche Nimblegen (Roche NimbleGen, Madison, WI) arrayCGH custom design was prepared using a layout of 720,000 unique oligonucleotides (50–60 mers) with average probe spacing across the X chromosome of 50 bp in genic regions and 20 kb in intergenic regions. The design was generated using NCBI37 human genome build (hg19). This design will be made available upon request. Microarrays were hybridized, scanned and analyzed according to the manufacturer's instructions using a Roche Nimblegen microarray analysis platform.
Quantitative PCR and X-Chromosome Inactivation
Q-PCR reactions were performed in a Roche Light Cycler 480 using the LightCycler FastStart Reaction Mix (Roche) with 10 ng of genomic DNA and 0.2 mM of each primer in a final volume of 20 μl. Only pathogenic and potentially pathogenic CNVs were confirmed by this method. Primer sequences are available upon request. The XCI status was determined using the DNA of the mothers of affected patients by methylation sensitive PCR as previously described [Villard et al., 2001].
RESULTS
The DNA of 54 male patients with XLID was analyzed by CGH using our custom X-chromosome array and 65 CNVs were identified. In order to classify these CNVs according to their putative pathogenicity, we have adopted a strategy allowing formulation of three rearranged groupings: probably benign CNVs (n = 60), pathogenic CNVs (n = 2), and potentially pathogenic CNVs (n = 3) (Fig. 1).
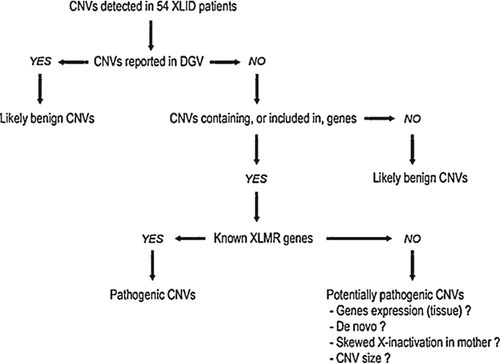
Probably Benign CNVs
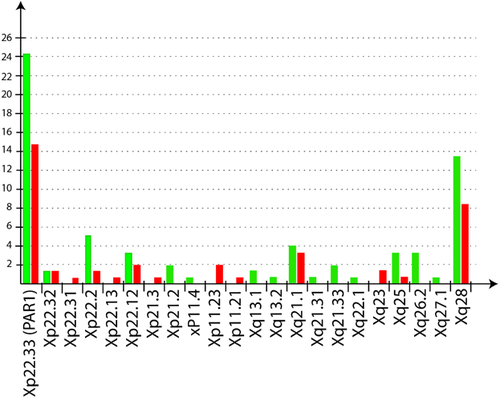
According to our classification scheme, probably benign CNVs are those that were already reported in an identical or overlapping region in at least two unrelated individuals, using non-BAC arrays, in the Database of Genomic Variants (DGV, http://projects.tcag.ca/variation/). We hypothesize that these CNVs are “probably” benign because DGV does not provide the gender of the individuals in whom the same or overlapping CNVs was/were identified. Given our X-linked focus, the pathogenic effect of a CNV can be masked by XCI in female carriers. We classified in that same category the CNVs that are located in intergenic regions or at more than 5 kb from the first exon of a gene to take into account a possible long-range effect on the promoter of neighboring genes. We also classified as probably benign the intronic CNVs located at more than 50 bp of splice sites on either side of an exon. The list of the CNVs falling in this category is provided in Supplemental Table SI (in Supporting Information Online). We identified 30 deleted regions, 13 duplicated regions, and 17 regions both deleted and duplicated in our cohort for this category of CNVs, with a size ranging from 140 bp to 104 kb. Interestingly, but not unexpectedly, we observed an overrepresentation of these so-called “benign” CNVs in distal Xp and Xq regions (Fig. 2).
Pathogenic CNVs
We found two CNVs involving genes whose mutations are already known to cause ID (Table I). The two CNVs were confirmed using Q-PCR with the patients' DNA. The first CNV is a 393 kb deletion involving exons 3–5 of the IL1RAPL1 gene in Xp21.3. This deletion is predicted to cause an in frame deletion of 207 amino acids (p.Asp29_Ala235del) from the 696 amino acids wild-type IL1RAPL1 protein.
| Locus | Aberration | Start (bp) | End (bp) | Size (bp) | Genes |
|---|---|---|---|---|---|
| Pathogenic CNVs | |||||
| Xp21.3 | Loss | 29029151 | 29422132 | 392,981 | IL1RAPL1 |
| Xq12 | Loss | 67411238 | 67415257 | 4,016 | OPHN1 |
| Potentially pathogenic CNVs | |||||
| Xp22.11 | Loss | 24020361 | 24042839 | 22,478 | KLHL15 |
| Xq23 | Loss | 111182217 | 111229432 | 47 215 | TRPC5 |
| Xq26.3 | Loss | 135300389 | 135302097 | 1,709 | MAP7D3 |
- Start/end positions are the position of the first/last deleted probe on the array. Position is based on UCSC Hg19.
The patient carrying this CNV was the first child of healthy unrelated 28-year-old mother and 52-year-old father (Supplemental Fig. S1A in Supporting Information Online). A maternal half sister was healthy whereas a maternal half brother was said to have psychomotor delay. Pregnancy and delivery were uneventful. Delivery occurred at 40 weeks gestation with a birth weight of 3,360 g (50th centile), length of 50 cm (30th centile), and 35.5 cm head circumference (85th centile). Strabismus and myopia were diagnosed soon after birth. He was referred for genetic evaluation at age 3.5 years because of significant motor and language delay. Walking was acquired at 22 months and he had no speech at 3.5 years of age.
Clinical examination revealed microcephaly (head circumference 48 cm, <5th centile), high frontal hairline, anteverted nares, short and upturned philtrum, thick vermilion of the lips with everted lower lip. The mother of the patient was found to carry the same CNV and had a completely skewed pattern of XCI. The second pathogenic CNV is a 4 kb deletion of exons 13–15 of the OPHN1 gene. This deletion will cause a frameshift with a loss of 58 amino acids (pI370_F427delfsX24) from the wild-type OPHN1 protein.
The patient was born at term after an uneventful pregnancy with normal weight, length, and head circumference. Both parents are mildly disabled. Family history showed that the maternal grandmother had two half-brothers suffering from ID (Supplemental Fig. S1B in Supporting Information Online). The neonatal period was unremarkable. He was first referred for examination in the first year of life because of global developmental delay. Febrile seizures were reported. At three years of age, walking was not achieved and the boy had no speech. Neurological examination showed spasticity and manual stereotypies. He had facial dysmorphism with prominent metopic suture, deep-set eyes, thin upper lip vermilion, abnormal external ears, and high frontal hairline. He had bilateral single palmar crease. Cerebral MRI showed cerebellar vermis hypoplasia with moderately dilated ventricles. Paternal DNA was not available for study. XCI ratio was found to be 70:30 in maternal DNA.
Potentially Pathogenic CNVs
According to the criteria defined by Isrie et al. [2012], three CNVs were considered to be potentially pathogenic (Table I). These CNVs were not previously identified in more than one “control” individual in our or other public cohorts and were involving genes highly expressed in the brain.
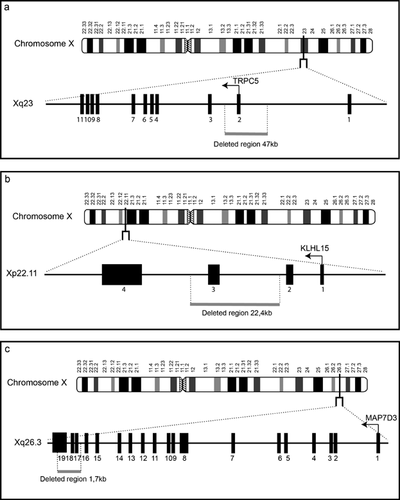
Deletion of TRPC5
We found a 47 kb deletion in Xq23 involving the first coding exon of the TRPC5 gene (Fig. 3a). There is no CNV affecting this region in public databases. The patient carrying this CNV was the first child of a healthy 19-year-old mother. No information was available about the father. Family history revealed an intellectually disabled grand maternal uncle (Supplemental Fig. S1C in Supporting Information Online). Macrocephaly was diagnosed during the third trimester of pregnancy. Fetal karyotype was normal. Uneventful delivery took place at 38 weeks gestation. Birth measurements were in the normal range with a weight of 3,550 g (75th centile), length of 50.5 cm (75th centile), and 35.5 cm head circumference (90th centile). Psychomotor development was delayed with walking achieved at 2 years. At 3 years of age, he was referred to a geneticist for speech delay and behavioral problems with frequent outbursts and self-injury. Autistic behavior was noted with respect to stereotypies and frequent withdrawal. On clinical examination, there were no dysmorphic features and neurological examination was normal. The deletion was also identified in the unaffected mother who displayed random XCI (50:50) in her lymphocytes.
Deletion of KLHL15
The second potentially pathogenic CNV was a 22 kb deletion in Xp22.11 involving the first three exons of the KLHL15 gene (Fig. 3b). This gene has four exons with two non-coding exons followed by two coding exons. This male proband was born to mildly disabled parents. Two brothers of the maternal grandmother were said to have ID (Supplemental Fig. S1D in Supporting Information Online). Pregnancy was uneventful. Delivery took place at 34 weeks gestation. Birth weight was 2,900 g (90th–95th centile). Length and head circumference were not recorded. Psychomotor development was delayed with walking achieved at 2 years of age and no speech at 4 years. Epilepsy was diagnosed at 13 months. Clinical examination revealed coarse facial features, anteverted nares large mouth, short hands, and abnormal genitalia with micropenis and bilateral cryptorchidism. Brain MRI performed at 23 months of age demonstrated polymicrogyric-like appearance of the central areas and parietal lobes predominantly right sided, with mild enlargement of the lateral ventricles and underdeveloped white matter mostly of the temporal areas. The mother was found to carry the deletion and to have skewed XCI in lymphocyte DNA (90:10). Sanger sequencing of the coding exons of the KLHL15 gene in 16 patients with various forms of polymicrogyria did not lead to the identification of additional mutations.
Deletion of MAP7D3
This CNV is a 1.7 kb deletion of the last three exons (exons 17–19) of the MAP7D3 gene in Xq26.3 (Fig. 3c). There is no known CNV in this region. This deletion affects the region of the gene encoding the last 47 amino acids of the MAP7D3 protein (pArg829_Gln876del according to ENST00000316077). This CNV was found in the patient carrying the OPHN1 deletion described above. (We were not granted permission to publish the photos of the patients.)
DISCUSSION
Determining the clinical relevance of X-inked CNVs is particularly challenging with regard to the potential consequences on genetic counseling, carrier detection, and possible prenatal diagnosis/preimplantation genetic diagnosis (PGD). CGH analysis in our series of 54 patients identified 65 CNVs scattered along the whole X-chromosome. We observed an over-representation of these CNVs in telomeric regions (Xp and Xq) containing 61.2% of the identified CNVs (38% for deletions, 23.2% for duplications). There is no relationship between the size of the chromosomal region and the number of CNVs that it contains.
The rate of pathogenic or potentially pathogenic CNVs identified in the present study is lower than the one reported in previous studies using the same approach. This is likely due to the fact that most patients included in our study had prior whole genome a-CGH. Therefore, large rearrangements such as the frequent MECP2 [Van Esch et al., 2005] or FMR1 [Rio et al., 2010] duplications that represent the majority of pathogenic CNVs in other series were not included here.
Two (4%) of the CNVs identified in this study were considered as likely pathogenic because they are affecting genes already known to cause XLID. In these two families, genetic counseling, carrier screening and prenatal diagnosis/PGD can be offered to parents and mother's relatives. The first CNV is an intragenic deletion of IL1RAPL1. IL1RAPL1 mutations were first described in 1999 [Carrié et al., 1999]. Intragenic deletions including two cases with deletions similar to the one described here and affecting exons 3–5 of the gene have been reported in association with non-syndromic or syndromic ID, autism, and isolated behavioral problems [Carrié et al., 1999; Nawara et al., 2008; Behnecke et al., 2011; Piton et al., 2011; Barone et al., 2013]. Dysmorphic features were reported in several patients but there seems to be no consistent facial gestalt. The patient reported here had severe microcephaly, whereas Patient 2 described by Behnecke et al. [2011] had macrocephaly.
The other causative CNV identified here is an intragenic deletion of the OPHN1 gene. Mutations in OPHN1 cause XLID with cerebellar hypoplasia and distinctive facial appearance. The severity of ID ranges from moderate to severe with intrafamilial variability. Several OPHN1 deletions were reported although none was affecting the same exons [Bergmann et al., 2003; Zanni et al., 2005; Madrigal et al., 2008; Al-Owain et al., 2011; Santos-Rboucas et al., 2014]. The deletion reported here was predicted to disrupt the reading frame and thus led to a complete absence of the protein. The patient's phenotype, including the facial features, anomalies of the posterior fossa and developmental delay, was consistent with the more severe end of the spectrum of OPHN1-related phenotypes. Interestingly, this boy had also a deletion affecting MAP7D3, which was considered as a potential candidate for ID. The MAP7D3 gene encodes a microtubule-associated protein, expressed in the mouse muscle, lung, and brain [Sun et al., 2011]. Several microtubule-associated proteins were shown to play a pivotal role for neuronal migration [Poulain and Sobel, 2010] and mutations in several members of this protein family cause various neurological phenotypes [Gillardon, 2009; Rovelet-Lecrux et al., 2009; Xie et al., 2011]. It is unclear whether this additional CNV plays a role in the severity of the clinical picture of this child.
Three new CNVs were considered as potentially pathogenic as they are affecting interesting candidates for ID. These three genes (TRPC5, KLHL15, and the MAP7D3 gene discussed above) are expressed in brain but have not been implicated so far in XLID. TRPC5 encodes a Transient Receptor Potential Cation channel (Subfamily C, Member 5). This gene is predominantly expressed in the developing and adult brain [Sossey-Alaoui et al., 1999] and is located in a chromosomal region where a non-syndromic intellectual disability syndrome (MRX35) has been linked [Gu et al., 1996; des Portes et al., 1999]. Recent studies have demonstrated the role of TRPC5 in neuronal development, particularly on dendritic growth and patterning [Freichel et al., 2005; Shin et al., 2010; Puram et al., 2011; He et al., 2012; Heo et al., 2012; Kaczmarek et al., 2012]. Mice lacking Trpc5 have reduced LTP [Phelan et al., 2013] and diminished innate fear levels in response to aversive stimuli [Riccio et al., 2009] but exhibit normal spatial learning [Phelan et al., 2013]. The brain-specific expression pattern makes TRPC5 a good candidate to cause an intellectual disability phenotype if abnormally expressed. The deletion involving the first coding exon is predicted to have a damaging effect for the protein encoded by this gene. KLHL15 encodes a Kelch-like protein that acts as an adaptor for the recruitment of substrates to E3 ubiquitin ligases. Not much is known about this protein but it was recently shown that KLHL15-Cul3 specifically targets the brain-specific regulatory subunit of PP2A, a major serine-threonine phosphatase [Oberg et al., 2012]. In both cases (TRPC5 and KLHL15 copy number variations) family segregation studies were not possible. Moreover, because these two genes are not expressed in lymphocytes or fibroblasts, we were not able to study their transcripts or proteins in patient cells thereby not providing additional evidence to support pathogenicity.
Recently, Piton et al., [2013] demonstrated that over interpretation of the pathogenicity of missense variants could lead to false positive conclusions about their involvement in XLID in a significant number of cases [Piton et al., 2013]. Further studies on mouse models or the identification of mutations in additional patients are now needed to confirm the involvement of TRPC5 and KLHL15 in XLID and before using this information for genetic counseling purposes.
ACKNOWLEDGMENTS
We thank Caroline Lacoste for performing X-chromosome inactivation studies and the Centre de Ressources Biologiques of Hôpital d'Enfants de la Timone (Karine Bertaux, Cécile Mouradian, and Andrée Robaglia-Schlupp) for assistance with the cell lines used in this study.