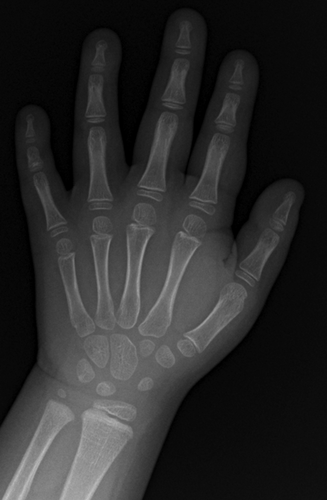
Advanced bone age in a girl with Wiedemann–Steiner syndrome and an exonic deletion in KMT2A (MLL)
Abstract
Recognition of the gene implicated in a Mendelian disorder subsequently leads to an expansion of potential phenotypes associated with mutations in that gene as patients with features beyond the core phenotype are identified by sequencing. Here, we present a young girl with developmental delay, short stature despite a markedly advanced bone age, hypertrichosis without elbow hair, renal anomalies, and dysmorphic facial features, found to have a heterozygous, de novo, intragenic deletion encompassing exons 2–10 of the KMT2A (MLL) gene detected by whole exome sequencing. Heterozygous mutations in this gene were recently demonstrated to cause Wiedemann–Steiner syndrome (OMIM 605130). Importantly, retrospective analysis of this patient's chromosomal microarray revealed decreased copy number of two probes corresponding to exons 2 and 9 of the KMT2A gene, though this result was not reported by the testing laboratory in keeping with standard protocols for reportable size cutoffs for array comparative genomic hybridization. This patient expands the clinical phenotype associated with mutations in KMT2A to include variable patterns of hypertrichosis and a significantly advanced bone age with premature eruption of the secondary dentition despite her growth retardation. This patient also represents the first report of Wiedemann–Steiner syndrome due to an exonic deletion, supporting haploinsufficiency as a causative mechanism. Our patient also illustrates the need for sensitive guidelines for the reporting of chromosomal microarray findings that are below traditional reporting size cutoffs, but that impact exons or other genomic regions of known function. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Several genetic syndromes have been associated with advanced bone age, including overgrowth syndromes (for example, Sotos syndrome, Beckwith–Wiedemann syndrome, and Simpson–Golabi–Behmel syndrome), syndromes that cause sexual precocity (for example, McCune–Albright syndrome), or other multiple congenital anomaly syndromes such as Marshall–Smith syndrome [Chen et al., 1993; Mieszczak and Eugster, 2007; Chen, 2012]. In patients with these diagnoses, advanced bone age is most commonly accompanied by an increase in linear height. Genetic causes of advanced bone age are otherwise poorly understood, particularly when associated with short stature.
The Mixed-Lineage Leukemia gene, MLL, was originally recognized as a component of somatic translocations causing particularly aggressive forms of acute leukemia [Muntean, 2013]. The HUGO Gene Nomenclature Committee (HGNC) approves the symbol KMT2A to refer to this same gene, and this symbol will be used throughout this report, though MLL remains commonly used in the literature. In 2012, Jones et al. reported that heterozygous, loss-of-function mutations in KMT2A were responsible for Wiedemann–Steiner syndrome (WSS) [Jones et al., 2012]. The five individuals in this study are, to our knowledge, the only cases in the literature with sequence-confirmed mutations in KMT2A [Jones et al., 2012], though additional cases have been published with a similar phenotype that likely represent the same syndrome [Koenig et al., 2010]. This syndrome is associated with dysmorphic facial features including a wide nasal bridge and nasal tip, Cupid's bow contour to the upper lip, long eyelashes, thick eyebrows, hypertelorism, and downslanting, narrow palpebral fissures. Developmental delay and short stature are also typical features. Hypertrichosis cubiti is a distinctive feature of WSS. Hypertrichosis cubiti can exist in isolation or in combination with the above anomalies, and was critical for identifying the cohort of patients to determine the molecular etiology of WSS [Beighton, 1970; Polizzi et al., 2005; Martinez de Lagran et al., 2010; Jones et al., 2012]. The phenotypic spectrum caused by KMT2A mutations may therefore be much broader if hypertrichosis cubiti is not a universal finding. Here, we report a young girl with a de novo, pathogenic mutation in KMT2A and many features of WSS who had marked hypertrichosis that did not disproportionately involve her elbows. A striking phenotypic feature was a severely advanced bone age despite short stature, suggesting that advanced bone age in the absence of overgrowth may be another important phenotypic clue as to a potential diagnosis of WSS. The causative mutation in this patient was an intragenic deletion in the KMT2A gene, despite her previous array comparative genomic hybridization (array CGH) study being reported as normal, making this the first report of an exonic deletion causing WSS and suggesting the need to consider modifying reporting criteria for array CGH.
CLINICAL REPORT
We present a female delivered by emergency Cesarean at 41 weeks gestation for an abnormal fetal heart tracing. Apgar scores were 2, 6, and 7 and 1, 5, and 10 min, respectively, and the first blood gas revealed a pH of 6.96 and blood lactate level of 9.2 mmol/L. She underwent a cooling protocol out of concern for hypoxic-ischemic encephalopathy due to acidosis and persistently poor spontaneous respiratory effort. She required intubation and meconium was noted beneath the vocal cords. Despite high frequency oscillatory ventilation, her respiratory status continued to decline and extra-corporeal membrane oxygenation (ECMO) was instituted from the first to the fifth days of life. Brain magnetic resonance imaging (MRI) several days after weaning from ECMO showed markedly decreased symmetric white matter volume loss and extensive thinning of the corpus callosum without evidence of hemorrhage. These changes were attributed to chronic hypoxic-ischemic injury. Also in infancy, a karyotype was reported as normal (46,XX).
At 2 months of life, a hip click was noted and an ultrasound evaluation of the hips revealed frank dislocation of the left hip with absence of the ossification center for the left femoral head and borderline dislocation of the right hip. She was treated with a Pavlik harness. Due to recurrent urinary tract infections, a renal ultrasound and a voiding cystourethrogram (VCUG) were obtained at 3 months of life that revealed grade IV vesicoureteral reflux and a left ureterocele. The ureterocele was punctured and she underwent ureter reimplantation at 1 year of life. Poor bladder tone and frequent urinary tract infections remain clinical concerns. She had severe gastroesophageal reflux and a Nissen fundoplication and gastrostomy tube placement was performed at 5 months of life. She continues to receive most of her nutrition by gastrostomy tube.
The patient was subsequently diagnosed with cerebral palsy due to global developmental delays, which were attributed to perinatal hypoxic-ischemic injury. However, tone and reflexes remained diminished, inconsistent with cerebral palsy, suggesting another etiology for the delay and white matter changes seen on her MRI. She walked at 17 months of age. At 4 years of age, she had developed expressive language in English and Spanish, though with immature articulation. She was not toilet-trained at this age. Her tone remained low but her strength was essentially normal. She was enrolled in special education for preschool and her teachers reported excellent progress, with mild learning difficulties. Brain imaging has not been repeated since infancy.
At 24 months of age, short stature was noted and a radiograph of the left hand was obtained to estimate the bone age, which was found to be approximately 2 years, 3 months according to the standards of Greulich and Pyle [1959]. At 3 years and 9 months chronological age, the bone age was repeated and was markedly advanced with an estimated age of 5 years and 9 months (standard deviation of 7.5 months). At 4 years and 3 months chronological age, another measurement was also markedly advanced with an estimated bone age of 6 years and 10 months (Fig. 1; standard deviation of 9 months). Her height at this time was 94.8 cm (0.85 centile) and body mass index 16.05 kg/m2 (73rd centile). A complete skeletal survey showed hypoplastic 12th ribs and a dysplastic left hip, but there were no additional abnormalities. A hormonal evaluation of her short stature and advanced bone age, including insulin-like growth factor binding protein-3 (IGFBP-3), insulin-like growth factor-1 (IGF-1), thyroid stimulating hormone (TSH), free T4, growth hormone, luteinizing hormone, estradiol, testosterone, and 17-hydroxyprogesterone was normal, and not consistent with thyroid or growth hormone dysfunction, precocious puberty, or excess sex steroids. Urine organic acids at this time were also normal.
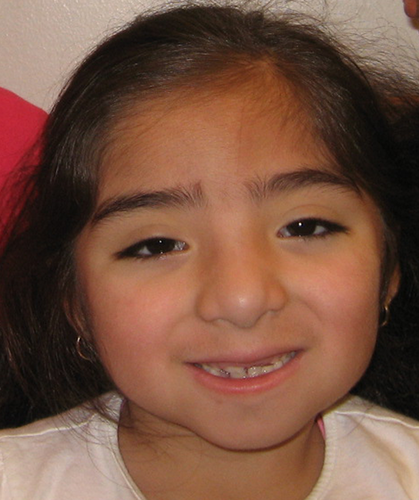
At 4 years of age (Fig. 2), her dysmorphic facial features included a prominent forehead with low anterior and posterior hairlines, thick eyebrows, mild ptosis, hypertelorism (interpupillary distance (IPD) was 6 cm; >97th centile), a wide nasal bridge and slightly low hanging columella and small ears (left ear was 4 cm; <3rd centile). She had several secondary teeth at 4 years of age. There was doughy and redundant skin on her hands and she had mild fifth finger brachyclinodactyly. There was marked hypertrichosis involving her back with a hair whorl, and her upper arms also had substantial hair growth, but no elbow hair was noted. Her tone and lower limb reflexes were mildly decreased. There were no signs of sexual precocity.
The family history did not reveal any known relatives with developmental delays, short stature, hypertrichosis, or similar facial features. Maternal and paternal ethnicity was Hispanic and consanguinity was denied.
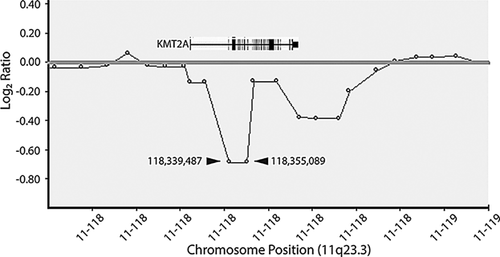
Array CGH was performed as a clinical test at 4 years of age using a 180K-feature whole-genome microarray (ISCA v1 Clinical Design; made by Agilent Technologies, Santa Clara, CA). Data were analyzed using BlueFuse software (BlueGnome, Cambridge, UK) and compared to copy number variation data from the Database of Genomic Variants (DGV, http://projects.tcag.ca/variation), the Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources (DECIPHER, http://decipher.sanger.ac.uk/), and the International Standards for Cytogenomic Arrays database (ISCA, https://www.iscaconsortium.org). A size threshold of 200 kilobases (kb) was used to report copy number gains and losses. This study was reported as normal. The patient was further investigated with whole exome sequencing that was performed as a clinical test using a trio approach (GeneDx; http://www.genedx.com), with accompanying samples from each parent. This study revealed a heterozygous, de novo, intragenic deletion involving the KMT2A (MLL) gene spanning exons 2–10 of the gene, establishing the diagnosis of Wiedemann–Steiner syndrome (WSS). Retrospective analysis of her microarray confirmed reduced copy number for two consecutive probes at chr11:118,339,487 and 118,355,089 (hg19), which are located within exons 2 and 9 of KMT2A, respectively, out of 36 exons (Fig. 3).
DISCUSSION
We report on a 4-year-old female with mild developmental delays, short stature, dysmorphic features that include hypertelorism, a prominent forehead with low anterior and posterior hairlines, mild ptosis, hypertelorism, a wide nasal bridge and low hanging columella and small ears. She had redundant skin on her hands and mild fifth finger brachyclinodactyly. Her back was hairy with a hair whorl and her upper arms also had marked hair growth. Her secondary dentition erupted early and she had several secondary teeth at 4 years of age. These dysmorphic features are in common with previously reported patients with mutations in KMT2A [Koenig et al., 2010; Jones et al., 2012]. This case of WSS includes organ system involvement that has not previously been described in past studies on this disorder. Koenig et al. reported advanced bone age in two patients with clinical features of WSS, including advancement by 3 months at age 2 3/12 years in one patient, and advancement by 4 months at age 6 8/12 years in a second patient [Koenig et al., 2010]. This degree of bone age advancement was not nearly as marked as in the patient reported here. Our patient also had renal anomalies including severe vesicoureteral reflux and a unilateral uretocele, and was diagnosed with congenital hip dysplasia, features not noted in previous case series. Patients with similar constellations of findings may warrant consideration of a diagnosis of WSS, and patients diagnosed with WSS by sequencing may benefit from imaging of the bones, hips, and kidneys.
We acknowledge that until further cases of WSS are described with these features, we cannot be certain that they are due to the KMT2A deletion. Other unknown genetic or teratogenic processes may be involved, but could be unrecognized or undetected by the patient's medical history, examination, and exome sequence. Indeed, further study of individuals with KMT2A mutations will be essential to fully define the phenotypic spectrum of WSS and provide guidance for appropriate medical screening of these patients.
Past reports of WSS with molecular diagnoses have all involved point mutations or deletions of one or four bases leading to premature stop codons, while our patient had a deletion of exons 2–10 from a total of 36 exons that includes the start codon [Jones et al., 2012]. We have not performed molecular analyses to determine if any transcription occurs from the mutant allele, such as from a distal in-frame ATG that may lead to a functional protein in which the N-terminus is truncated. It is possible that some residual KMT2A activity—normal or abnormal—is present in patients with alleles resulting in C- and/or N-terminal truncations that may account for some of the clinical variation.
An important feature of this case is the fact that the KMT2A deletion was detected by array CGH, but not reported because the deletion was too small (minimum size of 15.6 kb between the decreased probes), affecting only two probes. Array CGH is often employed as a first-line diagnostic tool in cases where a genetic condition is suspected [Miller et al., 2010]. The American College of Medical Genetics and Genomics (ACMG) recommends that microarrays provide a resolution of at least 400 kb, but specific guidelines relating to the reporting of copy number variants below this resolution are lacking and practices vary among medical centers and laboratories, professional organizations, and different geographic regions. Deletions or duplications below certain size cutoffs are routinely not reported with good reason, as the false positive rate and frequency of variants of unknown significance increases dramatically when smaller variants are considered [South et al., 2013]. Nevertheless, the genome is neither uniform in our understanding nor in the functional impact of a copy number variant, and reporting criteria should evolve to reflect this.
As array CGH technology advances, we recommend that attention be paid by national and professional organizations to creating reporting guidelines for deletions/duplications that affect coding regions that reflect the increased likelihood that such copy number changes may be pathogenic. In the short-term, possible improvements include denser probe coverage of exons on future array platforms, heightened attention at the level of the specialist interpreting an array CGH to probes with increased or decreased signal in consecutive exons, and/or a systematic approach for correlating deleted/duplicated exons and the patient's phenotype while reviewing array CGH output. Reflex testing to other methodologies better designed to detect single exon deletions/duplications may be a logical extension of traditional array CGH testing when small variants are detected. Eventually, array CGH may be more routinely coupled to complementary high resolution genome-wide screening modalities such as whole exome sequencing.
In summary, we report on the first case of WSS due to an exonic deletion of KMT2A and describe novel phenotypic characteristics, including a markedly advanced bone age, renal anomalies and variant patterns of hypertrichosis that should be evaluated in future cases of WSS. We also suggest attention to array CGH interpretation guidelines, both at the level of individual testing centers and international organizations, to improve the reporting of small deletions or duplications that involve coding sequence and thus could comprise mutations.