Expanding the clinical phenotype of patients with a ZDHHC9 mutation
Abstract
In 2007, 250 families with X-linked intellectual disability (XLID) were screened for mutations in genes on the X-chromosome, and in 4 of these families, mutations in the ZDHHC9 gene were identified. The ID was either isolated or associated with a marfanoid habitus. ZDHHC9 encodes a palmitoyl transferase that catalyzes the posttranslational modification of NRAS and HRAS. Since this first description, no additional patient with a ZDHHC9 mutation has been reported in the literature. Here, we describe a large family in which we identified a novel pathogenic ZDHHC9 nonsense mutation (p.Arg298*) by parallel sequencing of all X-chromosome exons. The mutation cosegregated with the clinical phenotype in this family. An 18-year-old patient and his 40-year-old maternal uncle were evaluated. Clinical examination showed normal growth parameters, lingual fasciculation, limited extension of the elbows and metacarpophalangeal joints, and acrocyanosis. There was neither facial dysmorphism nor marfanoid habitus. Brain MRI detected a dysplastic corpus callosum. Neuropsychological testing showed mild intellectual disability. They both displayed generalized anxiety disorder, and the younger patient also suffered from significant behavior impairment that required attention or treatment. Speech evaluation detected satisfactory spoken language since both were able to provide information and to understand conversations of everyday life. Occupational therapy examination showed impaired visual-spatial and visual-motor performance with poor drawing/graphic skills. These manifestations are not specific enough to guide ZDHHC9 screening in patients with ID, and emphasize the value of next generation sequencing for making a molecular diagnosis and genetic counseling in families with XLID. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
ID is the most prevalent severe handicap in children, affecting 1–3% of the population, and can be caused by environmental and/or genetic factors [Ropers, 2010]. The high degree of heritability of ID has been highlighted. Genetic causes include various chromosomal rearrangements diagnosed by conventional or array-based techniques, with a broad range of prevalence, as well as numerous autosomal or X-linked genes [Kaufman et al., 2011]. X-linked intellectual disability (XLID), which arises from mutations in genes on the X-chromosome accounts for about 10–12% of the ID seen in males [Ropers, 2010]. It is a genetically heterogeneous disorder with more than 100 genes currently described. In the course of systematic Sanger re-sequencing of the X-chromosome coding sequences in 250 families with XLID, 4 families were identified with pathogenic frameshift, splice-site or missense mutations in ZDHHC9 at Xq26.1. All mutations affected highly conserved amino acid (aa) residues of the corresponding protein. In three of the families, the ID phenotype was associated with a marfanoid habitus, although none of the affected individuals met the Ghent criteria for Marfan syndrome. Non-specific ID was diagnosed in the fourth family [Raymond et al., 2007; Raymond et al., 2009].
ZDHHC9 is highly expressed in the kidney, skeletal muscle, brain, lung, and liver [Swarthout et al., 2005]. It encodes a palmitoyltransferase that catalyzes the posttranslational modification of NRAS and HRAS [Swarthout et al., 2005; Rocks et al., 2005, 2006]. The degree of palmitoylation determines the temporal and spatial location of these proteins in the plasma membrane and Golgi complex [Swarthout et al., 2005; Raymond et al., 2007]. Since 2007, no additional mutations in ZDHHC9 have been reported in the literature, and there is currently not enough clinical information to determine which group of patients should be screened for this gene. Here, we report a family with a nonsense mutation in ZDHHC9 and provide detailed clinical findings including neuroradiological and neuropsychological assessments.
PATIENTS AND METHODS
Patients
Patient IV-2 (Fig. 1A) of family P148 was 18 years old at the time of last evaluation. He was born at term and the pregnancy was uneventful. Measurements at birth were weight 3,520 g, height 50 cm and occipito-frontal circumference (OFC) 37 cm. Given his family history, he was rapidly described as too quiet, with a poor interest in food and his environment. He was hospitalized at age 9 months for a syncope with hypotonia associated with fever, but febrile seizures were ruled out. Psychomotor delay was rapidly noted; walking was acquired at 24 months. The first words were pronounced at around 4 years, and the association of two words at around 5 years. He was able to utter sentences at around 11 years following speech therapy. He had sleep disturbance from the age of 8 months to 6 years, with a wake-up before 5:00 a.m. Since 2 years of age, he suffered from generalized anxiety, social phobia, depression, opposition, anger, and aggressive behavior (International Classification of Disease [ICD] 10), treated by risperidone and sertraline. He no longer had sleep disturbances in late infancy, but still had little interest in food. He was also treated for seizures with valproic acid between age 4 and 15 years, following an epileptic episode with centrotemporal spikes. Apart from some ear, nose, and throat infections, he was not ill often. He was moved to a school for special needs at age 9 years. His severe anxiety excluded work in an unprotected environment. Clinical examination showed normal growth parameters. He measured 177 cm, his arm span was 178.5 cm and his OFC 56.5 cm. Neurological examination showed lingual fasciculation, with no other signs of degeneration of anterior horn cells. A slightly reduced arm swing was noted, as well as difficulties in inflating the checks. Extraneurological manifestations included limited extension of the elbows (Fig. 2C) and metacarpophalangeal joints and moderate acrocyanosis of the hands. There was no facial dysmorphism or marfanoid habitus except for a long and thin appearance (Fig. 2A). Cerebral MRI detected a dysplastic corpus callosum (Fig. 2E–F).
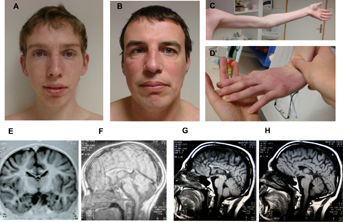
Patient III-3 is the maternal uncle of Patient 1 (Fig. 1A) and was 40 years old when evaluated. The pregnancy was uneventful, but with no regular medical care and home delivery. Psychomotor delay and hypotonia were rapidly noticed, and walking was acquired at age 2 years. At age 3 years, he only said a few words. He was transferred to a school for special needs at age 6 years. He was diagnosed with attention deficit disorder. He later worked in a protected area. He suffered from social phobia and generalized anxiety disorder (ICD 10), but showed no aggressive behavior. He is described as happy and easy-going. No history of frequent infection or seizures was reported. Clinical examination showed normal growth parameters. He measured 167 cm and his arm span was 175 cm and OFC was 58 cm. Neurologic examination detected isolated lingual fasciculation, a clearly reduced arm swing, and like for his nephew, it was impossible for him to inflate his checks and to move his tongue up and down. Similar extraneurological features were also identified: limited extension of the elbows and metacarpophalangeal joints and acrocyanosis of the hands (Fig. 2D). There was neither facial dysmorphism nor marfanoid habitus (Fig. 2B). He displays strabismus. Cerebral MRI detected a dysplastic corpus callosum (Fig. 2G–H).
Patient III-4, the brother of Patient 2, was described with severe ID, secondary to neonatal injury. He could not speak nor walk. He died at age 30 years of respiratory infection.
METHODS
Genetic Studies
For next generation-sequencing and genetic analysis, DNA was extracted from peripheral blood samples. Genomic DNA (3 µg) from the index patient (IV-2 in Fig. 1A) was used to construct a single-end Illumina sequencing library using the Illumina Genomic DNA Single End Sample Prep kit, according to the instructions of the manufacturer. For X-chromosome exome enrichment, we used the Agilent SureSelect Human X Chromosome Kit, which contains 47,657 RNA baits for 7,591 exons of the human X chromosome. Single end deep sequencing was performed on the Illumina Genome Analyzer GAIIx. Read-length was 76 nt. Sequences were analyzed with in-house-developed tools. Confirmation of the ZDHHC9 mutation and segregation analysis in the family was done by PCR and conventional Sanger sequencing using a gene-specific primer pair that flanked the mutated exon.
X-Inactivation Assay in Female Patients
This assay was performed as previously reported [Allen et al., 1992], with some slight modifications, notably the use of fluorescent primers and the detection mode. X-inactivation was considered as biased when it was superior or equal to 85%.
Neuropsychological Investigations
Two types of evaluation were used for each of the subjects: an evaluation of global intellectual performance comprising four sub-domains (verbal, non-verbal, working memory, and processing speed), as well as an evaluation of autonomy in four domains (communication, autonomy in everyday life, socialization, and motor skills). The WAIS-III scale [Wechsler, 1997] was used in both patients. The Progressive Matrices Standards of Raven (PMS-T) were also used in Patient 1 to evaluate non-verbal problem solving [Raven et al., 1998]. The Vineland Adaptative Behavior scale (VABS) interview was completed by the mother for Patient 1, and by the sister for Patient 2 [Sparrow et al., 1984].
Speech Evaluation
For Patients IV-2 and III-3, spoken and written languages were evaluated. Given their cognitive and linguistic performance, we used tests that assessed language development designed and standardized for infants/adolescents. The evaluation of spoken language concerned different aspects, including phonology, vocabulary and syntax; reception and expression. The evaluation of written language assessed the ability to understand a written text, and then reading and writing skills when possible. The following scales were used: PPVT (Peabody Picture Vocabulary test-revised) with the French version EVIP (Echelle de vocabulaire en images Peabody) [Thériault-Whalen and Dunn, 1993]; BALE, Bilan analytique du langage écrit (analytical assessment of written language) [Bosse et al., 2005]; ELO (Examen du Langage Oral) [Khomsi, 2001]; Le test de l'Alouette [Lefavrais, 1965 (revised 2006)].
Occupational Therapy Evaluation
Different tests were performed, including the Edinburgh Handedness Inventory, to determine dominance of one hand or the other, and the Developmental Test of Visual Perception, which comprises two parts: the MRP to evaluate visual-spatial function (indicating without using the hands) and the VMI (test of visual-motor integration involving use of the hands).
RESULTS
Genetic Studies
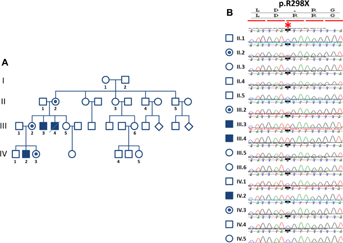
In an effort to identify the pathogenic mutations in families with XLID collected by the EUROMRX consortium and associated groups, we sequenced all X-chromosome specific exons in the index patient (IV-2 in Fig. 1A) of this large family (P148). After filtering of all identified variants against publicly available data, including the 1000 Genomes Project Database, dbSNP135 and the Exome Variant Server, the only one that was predicted to be deleterious was a nonsense mutation in ZDHHC9 (g.G>A, chrX: 128,944,967-128,944,967, UCSC, hg19, p.Arg298*). Subsequently, we confirmed the mutation by Sanger sequencing, and it co-segregated with XLID in the family (Fig. 1B). Indeed, the mutation was present in affected individuals III-1, IV-2 and unaffected obligate carriers II-6 and III-3, whereas it was absent in the healthy male family member IV-1 and the healthy females III-6 and IV-3 (Fig. 1B). X inactivation studies were inconclusive in II-2 and III-2 because of non-informative markers. Interestingly, haplotype analysis suggests that ZDHHC9 mutation have occurred in the germ line cells of I-2 individual (the father of the obligate carrier II-2).
Neuropsychological Investigations
For Patient IV-2, the results of the WAIS-III were homogeneous and showed mild intellectual disability (ICD 10 classification, processing speed index [PSI] = 54 [verbal IQ [VIQ] of 61, and performance IQ [PIQ] of 51], age equivalent: 9–11 years). Despite the overall deficiency, his verbal abilities were superior to his non-verbal abilities. For verbal abilities, Patient IV-2 was more successful in tests that involved social standards and concrete situations (experienced scenarios). He required a lot of encouragement. Concerning non-verbal abilities, the tests that assessed fluid intelligence (non-verbal abstraction) exceeded the pathological threshold and evaluation of visuoconstructive abilities showed difficulty with transferring a model in two dimensions to a configuration in three dimensions. The patient was also found to have an impaired working memory. He was completely unable to manipulate a small amount of information over a short period of time. The weakest performances concerned information processing speed (Pc < 0.1) which reflects impaired attention and short-term memory. The performances obtained with the Raven matrices were in keeping with the WAIS-III scores (Raw score: 16, Pc < 0.1, QI < 55). The results of the VABS showed age equivalents of 9½ years for communication, 13 years for socialization and 18 years 11 months for autonomy in everyday life. He achieved the maximum performance for motor skills (5 years 11 months). His autonomy in everyday life was clearly superior to his cognitive abilities (Tables I and II).
| Index | Percentile | 90% Confidence interval | |
|---|---|---|---|
| Patient IV-2 | |||
| Oral comprehension | 69 | 2 | 65–76 |
| Performance | 58 | 0.3 | 55–68 |
| Working memory | 56 | 0.2 | 53–65 |
| Processing speed | 50 | <0.1 | 49–65 |
| Patient III-3 | |||
| Oral comprehension | 65 | 1 | 61–72 |
| Performance | 56 | 0.2 | 53–67 |
| Working memory | 50 | <0.1 | 47–59 |
| Processing speed | 58 | 0.3 | 56–72 |
| Subdomain | Patient IV-2 | Patient III-3 | ||
|---|---|---|---|---|
| Raw score | Age equivalent (years–months) | Raw score | Age equivalent (years–months) | |
| Receptive | 25 | 4–8 | 22 | 2–6 |
| Expressive | 60 | 8–9 | 47 | 4–0 |
| Written | 33 | 9–4 | 8 | 5–7 |
| Communication | 118 | 9–6 | 77 | 4–4 |
| Personal | 78 | 17–6 | 73 | 8–9 |
| Domestic | 38 | 18–11 | 28 | 10.8 |
| Community | 56 | 18–3 | 49 | 14–9 |
| Autonomy | 172 | 18–11 | 150 | 11–9 |
| Interpersonal relationships | 48 | 14–3 | 48 | 14–3 |
| Play and leisure time | 32 | 11–6 | 28 | 7–11 |
| Coping skills | 33 | 19–9 | 34 | 15–0 |
| Socialization | 113 | 13–0 | 110 | 12–0 |
| Gross | 40 | 5–11 | 37 | 5–3 |
| Fine | 29 | 5–2 | 26 | 4–1 |
| Motor skills | 69 | 5–9 | 63 | 4–6 |
For Patient III-3, the results of the WAIS-III were homogeneous and showed mild intellectual disability (ICD 10 classification, PSI = 50 [VIQ = 53 and PIQ = 50], age equivalent: 9–11 years). In this patient, the verbal and non-verbal abilities were at the same level. For verbal abilities, the patient found it difficult to construct his discourse. His abilities in non-verbal analogical reasoning were also very limited and exceeded the pathological threshold. He was able to transfer a two-dimensional image into a three-dimensional configuration. However, he had difficulties with visual orientation. The patient's attention abilities and short-term and working memory were deficient. He was also easily distracted and very slow. The results of the VABS showed age equivalents of 4 years 4 months for communication, 12 years for socialization, 11 years 9 months for autonomy in everyday life, and 4 years 9 months for motor skills.
Speech Profile
With regard to spoken language, the profiles of Patients IV-2 and III-3 were similar, with relatively homogeneous scores in the different modules (vocabulary, syntax, and phonology). The language was informative, and comprehension for everyday life was satisfactory. There were no problems with either articulation or deformed words. The two patients therefore presented a profile of delayed language development, in keeping with their intellectual deficiency. In contrast, there was a difference between Patients IV-2 and III-3 with regard to written language. Even though the difference in reading comprehension age was small (Table I), the consequences were important. Patient 1 was able to identify words autonomously and was therefore able to understand meaning. He could also copy words and therefore use written language as a functional tool (Table III).
| Score | Age equivalent: years–month or school grade | Score | Age equivalent : years–month or school grade | |
|---|---|---|---|---|
| Spoken Language | ||||
| Articulation table | Repetition of logatoms (BALE) | |||
| Patient IV-2 | Normal | 18/20 | 4th year of primary school (9 years) | |
| Patient III-3 | Normal | Not tested | ||
| Vocabulary | Reception (EVIP) | Expression (ELO) | ||
| Patient IV-2 | Raw score = 110 | 10–4 years | 45/50 | 5th year of primary school (10 years |
| Patient III-3 | Raw score = 124 | 12–5 years | 38/50 | 5th year of primary school (10 years) |
| Syntax | Reception (ECOSSE) | Expression (ELO) | ||
| Patient IV-2 | 15 errors | 6–5 years | 22/25 | 5th year of primary school (10 years) |
| Patient III-3 | 11 errors | 7–5 years | 19/25 | 3rd year of primary school (8 years) |
| Written Language | ||||
| Reading (Alouette) | Dictation of nonsense words (BALE) | |||
| Patient IV-2 | Raw score = 72 | 6–10 years | 10/10 | 5th year of primary school (10 years) |
| Patient III-3 | Raw score = 22 | 6–6 years | Not-testable | |
| Dictation of regular words (BALE) | Dictation of irregular words (BALE) | |||
| Patient IV-2 | 7/10 | 2nd of primary school (7 years) | 2/10 | 2nd of primary school (7 years) |
| Patient III-3 | Not testable | Not testable | ||
Occupational Therapy Investigations
The results of the DTVP were as follows: visual perception index: 61 for Patient IV-2, and 64 for Patient III-3; visual-motor integration index: 46 for both patients. For the handedness test, patient IV-2 obtained a score of −3. There was no clear preference for the left hand. He could use the right hand for certain everyday activities. Patient III-3 obtained a score of +10, corresponding to a clearly dominant right hand. For the VMI, Patient IV-2 obtained a raw score of 64, corresponding to a standard score of 3. From a qualitative point of view, he was better able to draw oblique lines. He had a better representation of the overall shape and relationships between different elements. Patient III-3 obtained a raw score of 8, corresponding to a standard score of −1. He was unable to draw oblique lines and appreciate perspectives.
DISCUSSION
Here, we report for the first time on the presence of a nonsense ZDHHC9 mutation in association with non-syndromic ID. Only scarce clinical information is available in the literature regarding patients carrying a mutation in this gene. Indeed, no additional families with ZDHHC9 mutations have been reported since the first description in 2007 [Raymond et al., 2007]. There was no neuroimaging data and there was no information regarding specific skills. From the detailed clinical evaluation of the family described here, we demonstrate that patients had mild ID, had an anxious profile responsible for difficulties in their professional activity, and good skills for autonomy and understanding. As described in the previous publication by Raymond and colleagues, age at utterance of the first sentences was markedly delayed (Table IV). The better skills for autonomy of Patient IV-2 as compared to Patient III-3 from our family despite a similar intellectual level probably stems from the fact that Patient IV-2 benefited from better care from his earliest years. Since both patients display lingual fasciculation, and limited extension of the elbows and metacarpophalangeal joints, we believe that these features could be due to the ZDHHC9 mutation. Of note, limited extension of the elbows and metacarpophalangeal joints contrasts with joint hypermobility mentioned in the original paper [Raymond et al., 2007]. Similarly, although a dysplastic corpus callosum is not a specific finding, patients with XLID and dysplastic corpus callosum could be considered for ZDHHC9 screening. Since no neuroimaging data was available for the previously published families with a ZDHHC9 mutation, it is not known if an abnormal corpus callosum is a consistent finding or not. The clinical features of all reported families with ZDHHC9 mutations, including our family, can be found in Table I.
| Family | Mutation | Phenotype | Clinical features |
|---|---|---|---|
| 152 | c.172_175dup (p.Tyr59fsX33) | Non-specific ID | Normal measurements, hypotonia, cowlick, high forehead, transient solitary juvenile xanthogranuloma |
| No cerebral MRI available | |||
| 602 | c.167+5G>C (Thr11fsX33) | ID and marfanoid habitus | Walked at age 3½ years, limited speech at age 4½ years. Height 90th–97th percentile, arm span/height ratio 1.06, OFC >97th centile, pectus carinatum, pes planus, and arachnodactyly, thin facial features, major behavioral problems with schizophrenia |
| No cerebral MRI available | |||
| 031 | c.442C>T (p.Arg148Trp) | ID and marfanoid habitus | Feeding difficulties in infancy, walked at age 3 years, and talked at age 4 years, attended a special school, lived semi-independently in a supervised home. Normal measurements, flexion contractures of the elbows, large ears, long fingers and toes, pes planus |
| No cerebral MRI available | |||
| 576 | c.448C>T (p.Pro150Ser) | ID and marfanoid habitus | Pyloric stenosis at age 6 weeks. Transient adducted thumbs. Delayed sitting at age 12 months. Joint hypermobility, pectus excavatum, long digits, long face, strabismus, prominent ears, long, thin limbs with long digits, 5th-finger camptodactyly, long toes with camptodactyly. Little employment in adulthood, and height was in the 90th–98th percentile |
| No cerebral MRI available | |||
| Present report | c.892C>T (p.Arg298X) | Non-specific ID | Lingual fasciculation, limited extension of the elbows and metacarpophalangeal joints, anxiety, dysplastic corpus callosum |
The distribution of clinical presentations associated with ZDHHC9 is currently unknown. After the first description of ZDHHC9 mutations in patients with marfanoid habitus and ID [Raymond et al., 2007], we screened a cohort of 100 sporadic patients with the same presentation, and did not find a causative ZDHHC9 mutation in this patient group [Callier et al., 2013]. The introduction of next generation sequencing (NGS) in medicine will certainly answer these questions and highlight the clinical variability associated with each disease gene. However, thorough phenotyping of patients remains mandatory. As recently stated by Hennekam and Biesecker, diagnostic skills of medical specialists will shift from a pre-NGS-test differential diagnostic mode to a post-NGS-test diagnostic assessment mode. Indeed, when NGS is performed for diagnostic purposes, it will yield variants in several genes, and the consequences of these variants will need to be analyzed and integrated with clinical findings to make a diagnosis [Hennekam and Biesecker, 2012].
In conclusion, this report brings a detailed comprehensive description of a novel family with a pathogenic ZDHHC9 mutation. Such detailed clinical descriptions will be helpful in the future, in particular with the implementation of NGS.
ACKNOWLEDGMENT
The authors thank the family members for their participation in the study.




