Complex chromosomal rearrangements causing Langer–Giedion syndrome atypical phenotype: Genotype–phenotype correlation and literature review
Abstract
Langer–Giedion syndrome (LGS) is caused by a deletion of chromosome 8q23.3–q24.11. The LGS clinical spectrum includes intellectual disability (ID), short stature, microcephaly, facial dysmorphisms, exostoses. We describe a 4-year-old girl with ID, short stature, microcephaly, distinctive facial phenotype, skeletal signs (exostoses on the left fibula, coccyx agenesis, stubby and dysmorphic sphenoid bone, osteoporosis), central nervous system malformations (hypoplastic and dysmorphic corpus callosum and septum pellucidum), pituitary gland hypoplasia and hyperreninemia. Array-CGH revealed complex chromosomal rearrangements. A diagnosis of LGS was confirmed by the detection of a 8q23.3–q24.1 deletion. Associated chromosomal abnormalities were a 21q22.1 deletion and a balanced reciprocal translocation t(2;11)(p24;p15) de novo, confirmed by FISH analysis. We document the patient's atypical findings, never described in LGS patients, in order to update the genotype–phenotype correlation. We speculate that the disruption of regulatory elements mapping upstream CYP11B2 involved in the deleted region could cause hyperreninemia. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
Langer–Giedion syndrome (LGS) or trichorhinophalangeal syndrome type II (TRPS II) is a contiguous gene syndrome caused by a deletion of chromosome 8q23.3–q24.11. This deletion usually involves the EXT1 and TRPS1 genes [Pereza et al., 2012]. LGS includes the clinical features of two independent diseases: TRPS type I, caused by haploinsufficiency of TRPS1, and multiple hereditary exostoses (MHE) due to mutations/deletions in the EXT1 gene. TRPS type I is characterized by skeletal abnormalities (cone shaped phalangeal epiphyses, short stature), and craniofacial dysmorphisms (sparse, slowly growing scalp hair, bulbous-shaped nose, long flat philtrum, thin upper vermilion, large prominent ears). Cardinal features of MHE are multiple cartilagineous exostoses of long bones. Normal cognitive development is reported in both diseases [Shanske et al., 2008; Verheij et al., 2009].
RAD21 was recently found to cause Cornelia de Lange syndrome-4 (CdLs-4). CdLs-4 phenotype includes: short stature, synophrys, micrognathia, brachydactyly, vertebral anomalies and ID [Deardorff et al., 2012].
Isolated hyperreninemic hypoaldosteronism is frequently due to inherited defects of the aldosterone synthase, encoded by the CYP11B2 gene, mapping on chromosome 8q24 [Kayes-Wandover et al., 2001].
Here we describe a girl affected by LGS phenotype, carrying a microdeletion in 8q23.3–q24.13, a partial monosomy at 21q22.11 and a balanced translocation between chromosomes 2 and 11. This case represents the first report of a complex chromosomal rearrangement causing LGS; the patient presents with severe intellectual disability (ID), short stature, microcephaly, skeletal findings with exostoses, sphenoid bone malformation causing pituitary gland hypoplasia, central nervous system (CNS) malformations, and hyperreninemic hypoaldosteronism.
Speculation on the role of the genes involved in the chromosomal rearrangements is provided. We also discussed the need of an appropriate follow-up program for patients with LGS.
CLINICAL REPORT
The patient is the first child of healthy, unrelated parents. Both parents were of normal intelligence, without unusual facial appearance. She was born by caesarean section at 39 weeks of gestation. At birth the weight was 2.530 kg (5th–10th centile), length 46 cm (5th–10th centile), and head circumference 31 cm (5th–10th centile).
In the newborn period an echocardiography was performed, showing atrial septal and multiple small ventricular septal defects; spontaneous closure occurred; signs and symptoms of aldosterone synthase deficiency (ASD) were not evident.
Because of facial dysmorphisms, at 1 month of age, and later at 4 years of age the girl was referred to the Genetic Clinic Unit of the Department of Paediatrics, Federico II University of Naples. Clinical examinations revealed short stature, microcephaly, facial dysmorphisms (brachycephaly and low anterior hairline, synophrys, bushy eyebrows, down-slanting palpebral fissures, flat nasal bridge, long philtrum, thin upper lip vermilion, posteriorly rotated and protruding ears, overfolded helix, crease in the right ear lobe), hirsutism, short neck and short stature, telethelia, umbilical hernia, 5th finger clinodactyly of the feet (Fig. 1).
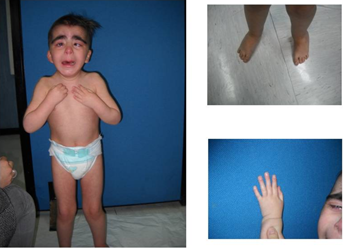
- - Brain MRI revealed: microcephaly, dysgenesis of corpus callosum (with agenesis of the rostro-caudal part) with evidence of Probst's bundle fibers; severly hypoplastic hippocampal commissure and septum pellucidum. Stubby and dysmorphic sphenoid bone (with sphenoid sinus agenesis), causing a shallow sella turcica with reduction in the anterior–posterior diameter, was identified. The pituitary gland was hypoplastic and dysmorphic and superiorly dislocated into the suprasellar cistern.
- - Skeletal X-ray revealed: delayed bone age, distal ulnar metaphysis dysplasia, left fibula exostosis, coccyx agenesis, 3rd and 4th metatarsal bone hypoplasia and absence of the middle phalanges of hands and feet in the middle fingers.
- - Calcaneal quantitative ultrasound densitometry and bone mineral density measured using dual-energy X-ray absorptiometry revealed marked osteoporosis (T-score −5.23, Z-score −3.68); 25-hydroxyvitamin D levels were 19 ng/ml (normal values 20–56 ng/ml).
- - Abdomen ultrasound revealed normal data.
- - Due to structural malformation of pituitary gland, endocrine evaluation (adrenal gland, thyroid, GH-IGF1 axis, prolactin, and sexual hormones) was performed, showing transient hyperthyrotropinemia and persistent hyperreninemia. Other laboratory evaluations showed fluctuating hyperkalemia (6.1–4.3 mmol/L range, median 5.2 mmol/L), persistent high plasma renin activity in the upright position (90, 34, 85.6, 53.5, 98.75, 154 pg/ml, normal values 0.9–20) and normal to mildly low aldosterone values (39, 66, 137.7 pg/ml normal values 70–300). ACTH, cortisol, 17-hydroxyprogesterone and blood sodium levels were within normal values in each assessment. Blood pressure was normal in multiple evaluations. ASD was diagnosed due to basal renin/aldosterone ratio (RRA), which resulted higher than 2 in multiple tests. Renal Doppler ultrasound revealed normal data.
On the basis of two episodes of simple febrile convulsions experienced by the child (tonic–clonic seizures), an EEG was performed and showed normal data. An assessment of psychomotor development at the age of 47 months was performed, using the Assessment Scale of Mental Development Griffiths (GMDS-R, 2–8 years). The developmental quotient was 36 corresponding to a severe developmental delay.
Since the complex phenotype of the patient raising CdLs, molecular analysis for NIPBL, SMC1A, and SMC3 genes was performed, showing no mutations. Due to the presence of ID, short stature, microcephaly, dysmorphic facial features and exostoses, a diagnosis of LGS was suspected (Table I).
| Cornelia de Lange syndrome | Our patient | Langer–Giedion syndrome |
|---|---|---|
| Microcephaly | Microcephaly | |
| Thick eyebrows | Thick eyebrows | Thick eyebrows |
| Arched eyebrows | Arched eyebrows | |
| Long philtrum | Long philtrum | |
| Thin upper lip | Thin upper lip | Thin upper lip |
| Prominent ears | Prominent ears | |
| Hirsutism | Hirsutism | |
| Short stature | Short stature | |
| Exostoses | Exostoses | |
| Congenital heart defects | Congenital heart defects | |
| Mild to severe cognitive impairment | Severe cognitive impairment | Mild to moderate cognitive impairment |
- The patient's phenotype partially overlapped to both LGS and CdLs typical features.
MATHERIALS AND METHODS
Metaphase chromosome analysis was performed on peripheral blood lymphocytes from the patient, and her parents, using high resolution G-banding (550 bands) according to standard procedures [Liehr et al., 2002]. An initial FISH analysis of derivative chromosomes detected in the patient was carried out with a whole chromosome paint (WCP) (MetaSystems, Altlussheim, Germany) and subtelomeric probes specific for chromosomes 2 and 11 (Vysis, Downers Grove, IL) according to manufacturer's instructions. Constitutional BoBs Kit and BoBsoft™ research analysis software (Perkin Elmer) was used to investigate LGS microdeletion syndrome. Array-CGH was performed with a cytochip Isca 4 × 44 Bluegnome whole-genome oligonucleotide array following the manufacturer protocol and the data were analyzed with BluFuse multi software. Real-time quantitative PCR for ASXL2 gene was performed using total RNA from proband and control whole blood and was reverse-transcribed using iScript cDNA Synthesis kit (Biorad, Milan, Italy). Real-time PCR was performed using iQ Supermix SYBR Green 2X (www.bio-rad.com) on the Bio-Rad iCycler (www.bio-rad.com) according to the manufacturer's protocols. PCR reactions were performed in triplicate. The primers (MWG Biotech, Ebersberg, Germany) used for amplification were: ASXL2-For CAGCATCTCTCACTAAAGACTGTCA; ASXL2-Rev CTTTCCTTCCCATGTTGCAG. Primer pairs were designed using the Primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi/) to obtain amplicons ranging from 100 to 150 base pairs. In order to test primer efficiency, serial dilutions of cDNAs generated from selected samples, that expressed target genes at a suitable level, were used to generate standard curves for each gene. GAPDH housekeeping gene was chosen as reference gene.
RESULTS
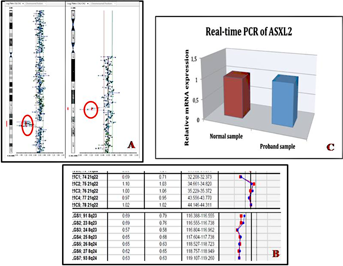
G-banded chromosomal and FISH analysis from the peripheral blood of the patient displayed a female karyotype with an apparent balanced reciprocal translocation t(2;11)(p24;p15) de novo (parental high resolution karyotype was normal); therefore, aCGH analysis was performed to detect the precise chromosomal rearrangement in this patient. Whole genome array-CGH revealed an interstitial deletion at 8q23.3q24.1 of about 7 Mb with breakpoints at 112,643,171 and 119,341,068 bp (Fig. 2A) and a deletion at 21q22.1 of about 760 kb between 32,952,375 and 33,710,645 bp. Seven genes are located in the 8q deleted region (National Center for Biotechnology Information, hg18) and 4 genes OMIM in the 21q22.1 monosomy region. The 8q and 21q deletion was confirmed by Constitutional BoBs (Perkin Elmer; Fig. 2B). Quantitative Real-Time PCR of ASXL2 gene showed normal expression level (Fig. 2C). We were interested in studying CYP11B2 expression in fibroblasts to investigate an impairment of CYP11B2 regulation but the patient's parents refused the skin biopsy.
DISCUSSION
In the present report we describe a female child with a phenotype suggestive for a LGS, confirmed by array-CGH analysis that revealed an interstitial deletion at 8q23.3–q24.1 (7 Mb). Cytogenetic studies also revealed 21q22.1 monosomy and a balanced reciprocal translocation t(2;11)(p24;p15). Within these multiple chromosomal abnormalities, the monosomy of 21q22 could be defined as a copy number variant, likely benign, due to: the unavailability of paternal array-CGH, the very small size (spans 708 Kb), its presence in databases of genomic variants. Moreover the de novo balanced chromosomal translocation does not seem to have any clinical consequence. Consequently only the 8q deletion seems to be related to the phenotype.
In addition to the typical LGS phenotype, the patient displayed distinctive skeletal findings and osteoporosis, CNS malformations, pituitary gland hypoplasia and hyperreninemia. The complex chromosomal rearrangements could explain some atypical manifestations although a specific genotype–phenotype correlation is hard to define.
Although deletions of 8q region are rare and considerably variable, the shortest region of deletion overlap (SRO) has been defined at 8q24.1, spanning 2 Mb, including TRPS1, EIF3S3, RAD21, OPG, CXIV, EXT1 genes [Lüdecke et al., 1991]. In our patient, the deletion at chromosome 8q includes the following genes: CSMD3, MIR2053, TRPS1, EIF3S3, UTP23, RAD21, MIR3610, C8orf85, SCL30A8, MED30, RPS10P16, EXT1, SAMD12. Recently, defects in RAD21 have been associated with CdLs-4. Although impressive and recognizable, face and hair of patients with LGS are variable and typically related to TRPS1 disruption [Shanske et al., 2008]. TRPS type I, LGS and CdLs-4 shared many facial features (Table I); some patients affected by LGS with deletions involving RAD21 and not TRPS1 present typical facial dysmorphisms (thick bushy and highly arched eyebrows, synophrys, prominent eyelashes, smooth philtrum, thin upper lip vermilion and hirsutism, Table II). LGS patients' facial hallmarks might be partially influenced by RAD21. Moreover the phenotypic consequences of RAD21 disruption might be underestimated in LGS patients (Table II).
| TRPS1 and CSMD3 deleted | TRPS1 and CSMD3 not deleted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Current case Del. 8q23.3–q24.11 | Tiberio et al. [1999] Del 8q23.2-q24.22 | Riedl et al. [2004] Del 8q24.1 | Carvalho et al. [2011] Del 8q23.1-q24.12 | Deardorff et al. [2012] (P4) Del 8q24.1 | Wuyts et al. [2002] Del 8q24.1 | Pereza et al. [2012] Del 8q23.3–q24.13 | Patient 3785 from Decipher Del 8q23.3–q24.12 | Deardorff et al. [2012] (P1) Del 8q24.1 | McBrien et al. [2008] Del 8q24.1 | |
| Short stature | + | + | + | + | + | − | − | + | + | |
| Microcephaly | + | + | − | + | + | + | + | + | + | |
| Thick eyebrows | + | + | + | + | + | + | + | + | + | |
| Synophrys | + | − | − | − | + | − | + | + | ||
| Thin upper lip and long philtrum | + | − | + | + | + | + | + | + | ||
| Skeletal abnormalities (exostoses, bone agenesis) | + | + | + | + | + | + | + | + | + | + |
| Reduced bone mineralization | + | |||||||||
| Hirsutism | + | − | − | + | + | − | − | |||
| Intellectual disability | + | − | ± | + | + | ± | ± | + | − | − |
| Brain malformation | + | + | − | |||||||
- The presence of TRPS1 and CSMD3 deletion is also considered.
- Empty cell = not available data.
Our patient shares the typical skeletal signs of LGS (brachydactyly, retarded bone age, cartilagineous exostoses, short stature) [Izumi et al., 2010]. Undescribed skeletal findings are: skull malformation (stubby and dysmorphic sphenoidal bone with sphenoid sinus agenesis, reduced sella turcica diameter) and coccyx agenesis. Mild skeletal features are present in patients with RAD21 haploinsufficiency without involvement of TRPS1, as reported in Table II. Moreover, the patient reported had a severe impairment of bone metabolism suggested by the presence of severe osteoporosis (T score: −5.23). Reduced bone mass is a component TRPS type I phenotype [Stagi et al., 2008; Izumi et al., 2010], while bone involvement seems to be uncommon in LGS patients.
Brain-MRI showed CNS morphological anomalies: interhemispheric connection structure malformations (dysgenesis of corpus callosum, hippocampal commissure and septum pellucidum hypoplasia), pituitary gland hypoplasia. In only two LGS patients, brain-MRI anomalies (symmetrical enlargement of lateral ventricles, focal T2 hypersignal at tuber cinereum) have been described [Wuyts et al., 2002]. Brain MRI was not performed in any other reported patient with LGS. To investigate putative etiologies of corpus callosum dysgenesis in the current patient, some possibilities have been evaluated. ASXL2, at 2p24.1, involved in CNS development, has been proposed to cause corpus callosum agenesis and cerebral malformations [Ramocki et al., 2003]. We evaluated ASXL2 mRNA levels that resulted normal, excluding its involvement in CNS malformations. Interestingly, microcephaly-thin corpus callosum syndrome was mapped at 8q23.2-q24.12 and RAD21 has been suggested as a potential candidate gene [Halevy et al., 2012]. RAD21 could be considered as a potential contributor to the corpus callosum dysgenesis and microcephaly.
Nearly 70% of LGS affected individuals may exhibit mild to severe cognitive disability [Wuyts et al., 2002]. Although delay in the LGS syndrome has been attributed to the deletion of genes outside the TRPS1-EXT1 interval, specific genes have to be identified yet. The size of the 8q deletion is directly correlated with ID [Riedl et al., 2004]. In the current patient, developmental delay is severe, the 8q deletion spans for about 7 Mb, and the genes potentially involved in development delay could be: CSMD3, MED30, SAMD12, EIF3H, RAD21. In particular CSMD3 has been previously suggested as a good candidate for pathogenesis of delay in LGS patients [Riedl et al., 2004]. RAD21 mutations or deletions have been observed in CdLs patients with very mild intellectual involvement [Deardorff et al., 2012]. Patients carrying 8q24 deletion, whose minimal overlapping interval involved RAD21 and not CSMD3 (see Table II) showed delay, demonstrating a possible role of RAD21. We hypothesized that RAD21, in combination with other genes, might contribute to the ID of LGS patients.
Moreover the patient showed high levels of plasmatic renin, inappropriately described low serum aldosterone levels, and normal value of cortisol and sex-steroid hormones. A diagnosis of ASD was hypothesized. ASD is a rare recessively inherited disorder responsible for failure to thrive, hypotension, hyponatremia and hyperkalemia in children. Two forms of ASD are recognized depending on profiles of secreted steroids [Kayes-Wandover et al., 2001; Løvås et al., 2009]. The clinical presentation of ASD varies with age. Infants might present with vomiting and dehydration in neonatal age, with failure to thrive, anorexia, mild dehydration and electrolyte abnormalities in childhood. Adults are usually asymptomatic [White, 2004]. The current patient showed elevated plasma renin activity, low or inappropriately normal aldosterone levels, normal cortisol levels, electrolytes abnormalities without any clinical symptoms and signs. Aldosterone synthase is a cytochrome P450 enzyme, which catalyzes the last three steps in the pathway of aldosterone biosynthesis and is encoded by CYP11B2, mapping at 8q24.3, telomeric to the deletion of the patient. Chromosomal deletions can alter the expression of neighboring genes also in 2–7 Mb away from breakpoints [Subhajyoti and Babu, 2010]. In the current patient the disruption of regulatory elements of CYP11B2 mapping at 8q23.3q24.1 might be hypothesized. Moreover the involvement of regulatory factors affecting CYP11B2 expression in five patients with isolated hyperreninemic hypoaldosteronism and negative for mutations in CYP11B2 gene, have been supposed [Kayes-Wandover et al., 2001]. The deletion of specific regulatory elements may lead to novel phenotypes, distinct from any identified for coding-region mutations. This would explain the atypical and mild ASD phenotype in the reported patient.
In conclusion we describe a patient with LGS diagnosis and complex chromosomal rearrangements consisting of a deletion at 8q23.3–q24.1 that confirm LGS, a 21q22.1 monosomy and a balanced reciprocal translocation t(2;11)(p24;p15). The patient, in addition to the typical features of LGS, showed atypical skeletal malformations and severe osteoporosis, CNS anomalies, pituitary gland hypoplasia, and hyperreninemia. RAD21 gene appeared to contribute to some facial features, skeletal involvement and brain anomalies. On the basis of our results, in patients affected by LGS, we suggest a brain and pituitary MRI for the investigation of morphological anomalies, X-ray surveys (for skull and spine malformations), DEXA for bone mineral density, and pituitary gland and adrenal function evaluations.




