CEP57 mutation in a girl with mosaic variegated aneuploidy syndrome
Abstract
Mosaic variegated aneuploidy (MVA) is a rare autosomal recessive disorder characterized by constitutional aneuploidies. Mutations in BUB1B and CEP57 genes, which are involved in mitotic spindle and microtubule stabilization, respectively, are responsible for a subset of patients with MVA. To date, CEP57 mutations have been reported only in four probands. We report on a girl with this disorder due to c.915-925dup11 mutation in CEP57, which predicts p.Leu309ProfsX9 and review the literature in order to facilitate genotype–phenotype correlation. Rhizomelic shortening of the upper limbs, skull anomalies with conserved head circumference, and absence of tumor development could be features suggesting a need for molecular screening of the CEP57 gene in patients with this disorder. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
Mosaic variegated aneuploidy (MVA; OMIM 257300, OMIM 614114) is a rare autosomal recessive disorder characterized by constitutional mosaic aneuploidies involving multiple distinct chromosomes and tissues [Hanks et al., 2004] and the frequency of aneuploidy is usually more than 25%. Premature chromatid separation (PCS) is another cytogenetic hallmark of MVA. Affected subjects manifest pre- and post-natal growth retardation, dysmorphism, and microcephaly. In addition, they display early-onset cancer predisposition to disorders such as leukemia, Wilms' tumor, or rhabdomyosarcoma. It has been shown that monoallelic or biallelic mutations in BUB1B “BUB1 mitotic checkpoint serine/threonine kinase B” and biallelic mutations in CEP57 “centrosomal protein 57kDa” genes, which are involved in mitotic spindle and microtubule stabilization, respectively, cause a subset of MVA [Hanks et al., 2004; Snape et al., 2011]. The BUB1B gene, located on chromosome 15q15, encodes a serine/threonine kinase called BUBR1, which makes key contributions to the coordination of chromosome segregation. The BUBR1 protein inhibits E3 ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C), which triggers chromosome segregation by targeting securin/separase complex [Tang et al., 2001]. The CEP57 gene, located on chromosome 11q21, encodes a centrosomal protein that localizes to microtubule networks, interacts with proteins in the microtubule cytoskeleton and is involved in intracellular trafficking [Emanuele and Stukenberg, 2007].
Since CEP57 mutations have been reported in very few patient [Snape et al., 2011] the description of an additional patients would be helpful to better delineate the phenotypic spectrum linked to this gene. Here we report on a girl with MVA due to mutation in CEP57 and review the literature in an attempt to delineate genotype–phenotype correlation.
CLINICAL REPORT
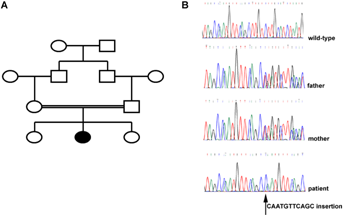
The proband, a 4-year-old Moroccan girl, is the second child of first cousin parents (Fig. 1). The parents had an otherwise unremarkable family history. The mother and the father were 22 and 29 years old, respectively at the time of the pregnancy. Ultrasound examination during pregnancy showed severe intrauterine growth retardation. A vascular etiology was suspected based on pathologic Doppler velocimetry. Amniocentesis performed at gestational week 27 showed a normal 46,XX karyotype (Table I). Delivery was normal at gestational week 37. Birth measurements were at −3 SD for weight and length (1.750 kg and 42 cm, respectively) and −2 SD for OFC (33 cm). The APGAR scores at 1, 5, and 10 min were 10, 10, and 10. No abnormal facial features were noted at birth.
| Age at study | Tissue sample | Number of metaphases | Range of chromosomes | Number of abnormal metaphases | Near-haploidy/near-diploidy (%) | Near-triploidy/near-tetraploidy (%) | Premature chromatid separation (%) |
|---|---|---|---|---|---|---|---|
| Prenatal | Amniotic fluid | 10 | 46 | 0 | 0 | 0 | 0 |
| 9 months | Blood | 53 | 42–53 | 6 | 11.2% | 0 | 0 |
| 10 months | Blood | 53 | 35–59 | 24 | 43.4% | 1.9% | 0 |
| 14 months | Blood | 100 | 23–92 | 32 | 22% | 10% | 0 |
| 14 months | Skin | 29 | 46 | 0 | 0 | 0 | 0 |
At 2 months, she was referred to the pediatrician due to poor growth of length and weight and a rapid increase in OFC. Her weight was 3.510 kg (−2 SD), length was 49 cm (−3 SD), and OFC was 38.5 cm (+0.5 SD). Psychomotor development and cerebral ultrasound were normal. Because of growth delay, routine cytogenetic analysis was performed on peripheral blood lymphocytes at 9 months. Results showed that 47/53 cells displayed a normal karyotype (Table I). Monosomy X was found in two cells, and near-diploidy in four cells. A second blood karyotype analysis at 10 months showed 29 normal cells (54.7%), 24 near-diplody/near-triplody cells (45.3%) for the 53 metaphases analyzed (Table I).
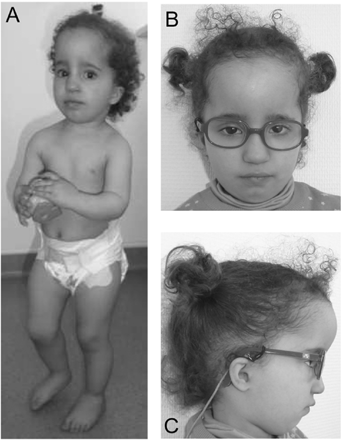
At the age of 14 months, she was referred to the Genetics Department. Examination showed sparse hair, prominent forehead with frontal bossing, dolichocephaly, deeply set eyes, closely spaced eyes, bilateral epicanthal folds, small and downslanted palpebral fissures, small and low-set round ears, small nose, small mouth, triangular face with micrognathia, and short neck (Fig. 2). Furthermore, she also presented with mild rhizomelic shortening of the upper limbs, bilateral single transverse palmar creases, and minor clinodactyly of the fifth fingers. The height was 64 cm (−4 SD), the weight was 7,500 kg (−2 SD) and OFC was 48 cm (+2 SD). Because of the dolichocephalic head shape, X-rays and CT scan were performed, and showed craniosynostosis with premature fusion of the sagittal and metopic sutures without signs of intracranial hypertension. Ophthalmologic examination, evoked visual potential and electroretinogram were normal. Cardiac, abdominal and renal ultrasounds, hearing tests, and thyroid function were also normal for age.
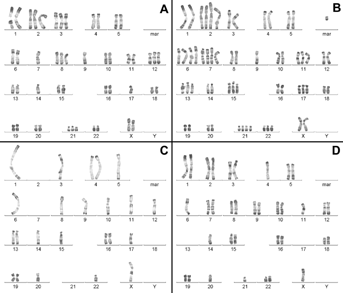
Cytogenetic analysis was subsequently performed on both lymphocytes and skin fibroblasts at the age of 14 months. All of the cytogenetic studies were done using standard RHG and GTG banding methods on metaphase chromosomes at the 550-band resolution level (Fig. 3). One hundred metaphases were analyzed from lymphocyte cultures and 32% showed abnormal chromosome number, being 22% near-haploidy/near-diploidy, and 10% near-triploidy/near-tetraploidy (Table I). Chromosomes involved in aneuploidy appeared to be random. Multiple trisomies (involving chromosomes 2, 3, 8, 11, 12, 13, 15, 17, 21, 22), tetrasomies (chromosomes 2, 6, 7), monosomies (chromosomes X, Y, 1, 3, 4, 6, 7, 9), and nullosomies (chromosome 2, 7, 13, 17, 21) were identified (Fig. 3). Furthermore, a few cells showed structural chromosome aberrations such as marker chromosomes (Fig. 3B). The cytogenetic analysis of 29 metaphase spreads obtained from skin fibroblasts was also performed. No aneuploidy was found notably due to the low number of metaphases analyzed. It is worth noting that no evidence of PCS was detected. The lack of PCS was also confirmed by fluorescence in situ hybridization using CEP X/18 probes (data not shown).
A survey combining physical, biological (including thyroid function), and ultrasound monitoring was performed every 3 months. As of this writing, there was no evidence of malignancy.
Based on cytogenetic data, the diagnosis of MVA was considered. We therefore screened the full coding sequence and intron–exon boundaries of BUB1B by direct sequencing. No mutation was identified (data not shown). We then sequenced CEP57 gene (primers and conditions are reported in Table SI). Using Sanger sequencing, we identified the homozygous 11-base pair insertion CAATGTTCAGC, c.915-925dup11, which predicts p.Leu309ProfsX9. This insertion was also present in the DNA in the heterozygous condition in both parents, supporting autosomal recessive transmission (Fig. 1).
DISCUSSION
When compared to the literature (Table II), the patient described here has clinical features previously reported in patients with CEP57 mutations and MVA, such as growth retardation with relative sparing of the head, and normal or mildly delayed development. The rhizomelic shortening of upper limbs was also observed in two other patients with CEP57 mutations and may be a specific feature. The patient reported here presented with craniosynostosis. This feature has not been described in the literature. However, some patients with MVA have been reported with frontal bossing and a conserved head circumference (Table II). We screened the most common genes involved in craniosynostosis in an attempt to identify a pathogenetic mutation. We did not find mutations in the FGFR2 (exons 4, 6, 8, 10, 11, 14, 15, 16, 17) or in the coding region of TWIST1. Furthermore, we did not find the common pathogenetic mutation within FGFR3 (p.Pro250Arg) (data not shown). It is interesting to note that FGF2 is one of the FGF2R ligands, which is mutated in 32% of patients with craniosynostosis [Iseki et al., 1999; Johnson and Wilkie, 2011]. Because CEP57 acts as FGF2 partner [Bossard et al., 2003], we suggest that CEP57 may be implicated in craniosynostosis.
| Case | 663_1 | 663_2 | 638 | 657 | Present case | Total |
|---|---|---|---|---|---|---|
| Age (years) | 8.5 | 4.5 | 3 wks (d*) | 15 (d**) | 4 | 3M/2F |
| Sex (M/F) | M | F | M | M | F | |
| Origin | Mexican | Mexican | Caucasian | Caucasian | Morrocan | |
| Clinical features | ||||||
| Intellectual disability | − | − | na | Mild | Mild | 2/4 |
| IUGR | − | + | + | − | + | 3/5 |
| Growth retardation | + | + | + | + | + | 5/5 |
| Microcephaly | − | + | − | + | − | 2/5 |
| Heart anomalies | − | − | + | Mild | − | 2/5 |
| Facial features | ||||||
| Skull anomalies | na | na | + (a) | + (b) | + (c) | |
| Deep set eyes | na | na | + | na | + | |
| Ears anomalies | na | na | na | na | + | |
| Small mouth/micrognatia | na | na | na | na | +/+ | |
| Rhizomelic shortening | − | − | + | + | + | 3/5 |
| Single palmar crease/clinodactyly | na | na | +/+ | na | +/+ | |
| Other features | − | − | Duodenal atresia | Hearing impairement | ||
| Hypotonia | Sleep apnea | |||||
| Biology | ||||||
| Hypothyroidism | + | − | na | + | − | 2/4 |
| Cytogenetics | ||||||
| Mosaic Aneuploidy | + | + | + | + | + | 5/5 |
| PCS | − | 14 % | − | na | − | 1/4 |
| Cancer | − | − | − | − | − | 0/5 |
| Mutations | c.520_521delGA | c.520_521delGA | c.241C>T | c.915_925dup11 | c.915_925dup11 | |
| c.915_925dup11 | c.915_925dup11 | c.241C>T | c.915_925dup11 | c.915_925dup11 | ||
| Reference | Snape et al. [2011] | Snape et al. [2011] | Snape et al. [2011] | Snape et al. [2011] | This report | |
- M, male; F, female; d*, died after cardiac surgery; d**, died after sleep apnea; wks, weeks; na, not available; (a), temporal bossing; (b), narrow head with sagittal ridge; (c), craniosynostosis with premature fusion of the sagittal and metopic sutures; PCS, premature chromatid separation.
The patient described here had not shown tumor development, as those previously reported did not [Snape et al., 2011]. This is in contrast with the observation that patients with BUB1B mutations have an elevated risk for developing cancer compared to the general population. However, this aspect deserves further investigation since none of the patients with CEP57 mutations and MVA have reached adulthood. Since few patients with MVA syndrome caused by CEP57 mutations have been reported, the careful description of additional cases is important to further delineate the phenotypic spectrum and confirm phenotype–genotype correlations.
In addition to the patient reported here, four patients with biallelic CEP57 mutations have been described in the literature [Snape et al., 2011]. One patient was homozygous for the nonsense mutation c.241C>T which predicts p.Arg81X. The other patients were homozygous or compound heterozygous for the c.915-925dup11 mutation (Table II). This finding suggests that the c.915-925dup11 mutation is more common than the other CEP57 mutations that cause MVA. The ethnic origin of the three published patients with the c.915-925dup11 mutation is not always well defined. Two of them (brother and sister) were of Mexican origin while the third was of Caucasian origin. These observations do not suggest a founder effect. The c.915-925dup11 mutation maps to exon 9, which is important for both microtubule localization and stabilization. It is likely that CEP57 mutations affect proper microtubule localization/stabilization, thus causing aneuploidy. Furthermore, CEP57 binds basic fibroblast growth factor (FGF2) and mediates its nuclear translocation and mitogenic activity [Bossard et al., 2003]. We speculate that these mutations could also interfere with nuclear translocation of FGF2 and the ability of FGF2 to stimulate proliferation thus leading to growth and development delay.
ACKNOWLEDGMENTS
This work was supported by grants from Associazione Italiana Ricerca sul Cancro (AIRC) and Istituto Toscano Tumori to A.M.