Recurrent compartment syndrome in a patient with clinical features of a connective tissue disorder†
Abstract
Arterial complications are common in vascular type Ehlers–Danlos syndrome (EDS), accounting for 66% of first complications. Several cases in the literature have documented acute compartment syndrome (ACS) following vascular rupture in vascular type EDS. Other disorders of connective tissue have also demonstrated vascular fragility, leading to arterial aneurysm and rupture, but there have been no documented cases of ACS. Here, we report on a female patient with a history of recurrent compartment syndrome who exhibits some clinical findings seen in hypermobile and vascular EDS; however she does not meet clinical and molecular diagnostic criteria for either of them. We further review the literature on ACS in heritable connective tissue disorders and suggest that compartment syndrome may rarely complicate other heritable disorders of connective tissue. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
Compartment syndrome refers to a condition of compromised circulation and function of the tissues within a limited space due to increased pressure within that space. The reduced tissue perfusion results in reduced venous drainage, leading to an increased interstitial tissue pressure and subsequent tissue edema, collapse of lymphatic vessels, and severely compromised arterial flow. Left untreated, acute compartment syndrome (ACS) can result in irreversible necrosis of muscle, infection, neurological deficit, acute renal failure, need for limb amputation, and even death [Matsen and Krugmire, 1978]. Diagnosis of ACS relies on a high index of suspicion based on the patient's presenting signs and symptoms which include pain that is out of proportion to the stimulus, altered sensation, pain, and palpable tenseness of the affected muscle compartment, and muscle weakness. Clinical diagnosis is of high importance because successful treatment weighs heavily on early diagnosis [Elliott and Johnstone, 2003].
Though there are many causes of compartment syndrome, most cases typically follow traumatic injury to a limb [Matsen and Krugmire, 1978]. Vascular injury, particularly arterial damage, is another important cause. The resulting hemorrhage following arterial injury rapidly increases compartmental pressures and leads to the characteristic sequelae of ACS [Suzuki et al., 2005].
Spontaneous arterial bleeding in an extremity may be the initial symptom in patients with vascular type Ehlers–Danlos syndrome (EDS type IV) [Schmalzried and Eckardt, 1992]. It is estimated that 1 in 100,000–250,000 people are affected by the vascular type of EDS, accounting for less than 4% of all EDS cases [Oderich et al., 2005; Dwivedi et al., 2012]. Vascular type EDS involves an autosomal dominant inherited genetic defect in COL3A1 resulting in defective or absent type III collagen. Type III collagen is a major component of connective tissues including blood vessel walls, skin, intestines, and uterus [Schmalzried and Eckardt, 1992]. As a result, 66% of patients affected by vascular type EDS present first with an arterial complication. Though complications are rare during childhood, by age 40 nearly 80% of patients have experienced some complication [Pepin et al., 2000].
Here, we report on a woman who presented with multiple episodes of spontaneous vascular rupture and compartment syndrome, who also had some clinical features of hypermobility syndrome (EDS type III). Biochemical and genetic testing excluded abnormalities in type III collagen. These observations suggest that compartment syndrome may complicate other heritable disorders of connective tissue.
CLINICAL REPORT
This female patient, now age 43, first presented to our clinic at age 37. Her medical history includes recurrent episodes of compartment syndrome, starting at age 27, culminating in an estimated 60–70 fasciotomies performed. All limbs have been affected including the left hand (three to four times), left arm (twice), left leg (twice), left foot (two to three times), right foot and calf (around 10 times), and the right hand (three times). Most episodes have occurred secondary to minor trauma although some of them were spontaneous. In six episodes, an elevated compartment pressure was documented. She has not had any other significant vascular or tissue fragility reported. She bruises easily and reports slow wound healing with normal scar formation. She reports being flexible as a child but has not had any joint dislocations. She has never been pregnant and has regular menstrual cycles that typically last 4 days with normal bleeding. She underwent an extensive workup for possible bleeding diathesis and immune dysfunction that was normal (Table I). The patient was adopted and family history is limited, although both her mother and maternal grandmother were reported to bruise easily. She is of Lithuanian descent and has two normal half-siblings. She has myopia and a recent echocardiogram was unremarkable. The patient is now experiencing chronic diffuse joint and soft tissue pain. She is currently on disability due to chronic debilitating pain following improper healing after the numerous surgeries that she has had for compartment syndrome. She is treated for depression and chronic pain with opiate analgesics. Pertinent findings on physical exam revealed normal body habitus with non-dysmorphic facial features (Fig. 1A). Palate was normal with a single uvula. Skin was velvety in texture without significant hyperelasticity. It was not transparent, and the venous pattern was not visible. She has multiple surgical scars, which are normal on all extremities (Fig. 2). Joint laxity was noted in the elbows and fifth fingers although full evaluation for Beighton score was limited because of joint pain triggered by motion. On the medial aspect of the right leg, an ulcer was noted that has not healed for more than 3 months (Fig. 1B). Based on these findings, the differential diagnosis was vascular EDS versus atypical hypermobility syndrome. Biochemical testing using patient-derived skin fibroblasts revealed normal synthesis, secretion, and electrophoretic mobility of type III collagen. Subsequently, sequencing of COL3A1 did not reveal disease causing mutations in the gene.
| Test |
|---|
| Prothrombin time (PT) |
| Partial thromboplastin time (PTT) |
| INR |
| Platelet count |
| Von Willebrand factor |
| Protein electrophoresis |
| Immunoglobulin assay (IgE) |
| SS-A antibody |
| SS-B antibody |
| SCL-70 antibody |
| Cold agglutinins |
| Anti-nuclear antibody (ANA) |
| C-reactive protein (CRP) |
| Complement: C3, C3PA, C4, Complement activity, C1 esterase inhibitor, C1 esterase inhibitor function |
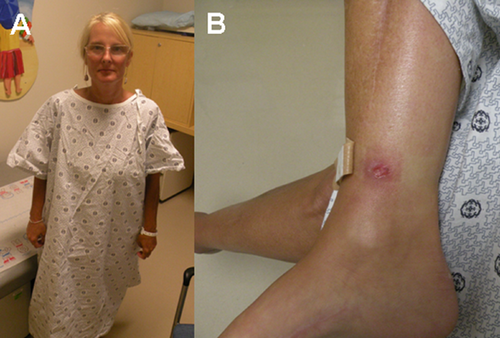
Physical findings in patient with recurrent compartment syndrome. A: Normal body habitus with non-dysmorphic facial features. B: Non-healing ulcer located superior to right medial malleolus.
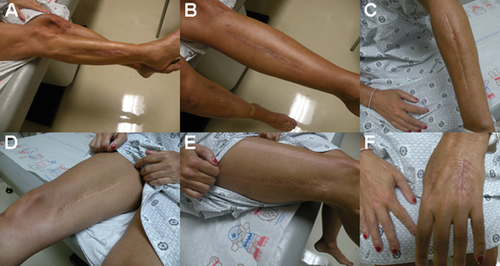
Surgical scars, following faciotomies. A: Lateral aspect of right leg. B: Anterior aspect of left leg. C: Left posterior forearm. D: Right medial thigh. E: Dorsum of left hand.
DISCUSSION
Compartment syndrome has been reported as a rare complication of vascular EDS. This report suggests that compartment syndrome may rarely complicate other heritable disorders of connective tissue secondary to generalized fragility of connective tissues including the blood vessel wall. Though the patient described here exhibited some features associated with hypermobility EDS (i.e., joint hypermobility, joint pain and mild skin findings), the vascular manifestations are not consistent with this form [Beighton et al., 1998].
Episodes of ACS may occur following trauma with or without a bone fracture as well as from non-traumatic causes. Bone fractures account for an estimated 75% of all cases of ACS. In the remaining quarter of cases, injury to soft tissue without fracture is responsible for causing ACS [McQueen et al., 2000]. In 1992, the first case of a patient developing ACS as a result of complications associated with vascular type EDS was reported [Schmalzried and Eckardt, 1992]. In this case, spontaneous bleeding from a gluteal artery resulted in a gluteal compartment syndrome and sciatic neuropathy. The diagnosis was made based on the patient's history of recurrent shoulder dislocations, bleeding from multiple arterial aneurysms, and supportive histopathology [Schmalzried and Eckardt, 1992]. Molecular and biochemical testing were not reported. Since this initial report, several cases of patients with vascular type EDS developing ACS have been documented [Oderich et al., 2005; Matsushima and Takara, 2009; Yoshimura et al., 2011]. One case involved a 47-year-old male who suffered bilateral renal artery dissection with visceral ischemia resulting in an abdominal compartment syndrome. He had clinical features consistent with vascular type EDS, and diagnosis was confirmed with biochemical and genetic testing [Oderich et al., 2005]. A second case involved a 27-year-old female with a known history of vascular type EDS who suffered spontaneous rupture of the posterior tibial artery resulting in compartment syndrome [Matsushima and Takara, 2009]. Lastly, a third case was reported in which a 33-year-old male patient presented with recurrent compartment syndrome from ruptured aneurysms of middle-sized arteries (an artery in left lower leg at age 31, left ulnar artery at age 33). Diagnosis of vascular type EDS was made based on clinical findings and confirmed by genetic testing [Yoshimura et al., 2011]. Although arterial fragility is a common finding in other connective tissue disorders including Marfan syndrome, Loeys–Dietz syndrome (LDS) and even in classic EDS (in a couple of recently reported cases), ACS has not been described in any of them [Borck et al., 2010]. In the case reported here, the patient did not present with any of the clinical features associated with these syndromes. Marfan syndrome, LDS, and vascular type EDS are all known to exhibit arterial aneurysms and/or dissections [Pepin et al., 2000; Loeys et al., 2005, 2010; Kalra et al., 2011]. In Marfan syndrome and LDS, aneurysms typically involve large-sized arteries like the aorta [Loeys et al., 2005, 2010; Kalra et al., 2011]. Vascular aneurysms in patients with vascular EDS typically affect middle-sized arteries; however, some individuals may develop aortic aneurysm and/or dissection [Oderich et al., 2005; Matsushima and Takara, 2009; Loeys et al., 2010; Yoshimura et al., 2011]. Though the exact mechanism for spontaneous ACS in our patient is not known, it is possible that they developed from a ruptured aneurysm. Unfortunately, vascular imaging studies have not been completed to confirm this possibility or to look for additional vascular abnormalities due to insurance deficit.
Vascular type EDS is clinically heterogeneous. Although some patients lack the classic external features, nearly all exhibit the internal arterial/bowel complications of aneurysms and/or spontaneous bleeding or rupture and show a deficiency of type III collagen [Sheiner et al., 1985; Gertsch et al., 1986; Cikrit et al., 1987; Shohet et al., 1987; Bellenot et al., 1990]. Presumably, the deficiency in type III collagen is due to a mutation in COL3A1, the gene that encodes for type III procollagen and the only gene that is associated with this form of EDS [Pepin et al., 2000]. Therefore, although the clinical features of the patient reported here are not distinctive of vascular type EDS, biochemical and genetic testing were obtained [Beighton et al., 1998; Pepin et al., 2000].
Our patient also exhibits some clinical findings characteristic of EDS, hypermobility type, namely hyperextensible joints, joint pain and velvety skin that bruises easily and heals slowly [Beighton et al., 1998]. Although arterial dilation has been reported in a few cases of hypermobile EDS, they are usually not progressive and are not associated with spontaneous rupture [Leier et al., 1980; Wenstrup et al., 2002; McDonnell et al., 2006].
CONCLUSION
We report recurrent compartment syndrome in a patient with some clinical features of an unclassified connective tissue disorder and suggest that recurrent compartment syndrome may complicate other disorders of connective tissue. The unique nature of this case may remind healthcare providers to suspect a connective tissue disorder in a patient who presents with compartment syndrome. Future investigations such as testing other collagen genes, imaging studies, or even whole exome sequencing despite limited availability of biological family members, may shed more light on the mechanisms underlying recurrent ACS in this patient.
ACKNOWLEDGMENTS
Dr. Rimoin received support from the Steven Spielberg Pediatric Research Center, the NIH/NICHD Program Project Grant (HD36657), the Medical Genetics NIH/NIGMS Training Program Grant (5-T32-GM08243), and the Cedars-Sinai General Clinical Research Center Grant (M01-RR00425).