Phenotypic variability in hyperphosphatasia with seizures and neurologic deficit (Mabry syndrome)†‡
Miles D. Thompson and Tony Roscioli are equal first authors.
How to Cite this Article: Thompson MD, Roscioli T, Marcelis C, Nezarati MM, Stolte-Dijkstra I, Sharom FJ, Lu P, Phillips JA, Sweeney E, Robinson PN, Krawitz P, Yntema HG, Andrade DM, Brunner, HG, Cole DEC. 2012. Phenotypic variability in hyperphosphatasia with seizures and neurologic deficit (Mabry syndrome). Am J Med Genet Part A 158A:553–558.
Abstract
Hyperphosphatasia with neurologic deficit (Mabry syndrome) was first described in a single family (OMIM#239300) by Mabry et al. [1970]. Although considered rare at the time, more than 20 individuals with the triad of developmental disability, seizures, and hyperphosphatasia have been identified world-wide. The 1-6 mannosyltransferase 2, phosphatidylinositol glycan V (PIGV) gene has been found to be disrupted in some patients with the additional feature of brachytelephalangy. In the present report we identify three patients compound homozygous for PIGV mutations. Two siblings were found to be compound heterozygotes for c.467G > A and c.494C > A in exon 3 of PIGV (the c.494C > A PIGV variant is novel). A third patient with similar phenotype, was a compound heterozygote for the known c.1022C > A/c.1022C > T (p.Ala341Glu/p.Ala341Val) mutation. This patient was also noted to have lysosomal storage in cultured fibroblasts. In contrast, the fourth patient who had no apparent hand abnormality, was found to be heterozygous for a previously unclassified c.1369C > T mutation in exon 4 of the PIGV gene, resulting in a p.Leu457Phe substitution in the catalytic domain of the enzyme. Unless this variant has a dominant negative effect, however, it seems likely that another GPI biosynthesis gene variant may contribute to the disorder, possibly through digenic inheritance. Since slightly fewer than half of the nine cases presented in this report and our previous report [Thompson et al., 2010] have PIGV mutations, we suggest that other genes critical to GPI anchor biosynthesis are likely to be disrupted in some patients. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Mabry syndrome manifests in the first year of life with developmental disability, seizures, and hyperphosphatasia. The elevation of tissue nonspecific alkaline phosphatase distinguishes Mabry syndrome from other causes of intellectual disability [Thompson et al., 2010]. The syndrome was first reported in three siblings and a first cousin from one family, and an autosomal recessive mode of inheritance was suggested [Mabry et al., 1970].
Originally described as the triad of developmental delay, seizures, and persistent hyperphosphatasia, the syndrome was not well delineated until recently [Thompson et al., 2010]. Five published studies of seven families with 21 affected individuals have appeared [Gomes and Hunter, 1970; Mabry et al., 1970; Kruse et al., 1988; Rabe et al., 1991; Cole and Whyte, 1997] prior to the recruitment of cases for the present study [Thompson et al., 2006]. Over 20 cases that broadly fit the original description of the syndrome have been recruited for molecular studies [Horn et al., 2010; Thompson et al., 2010].
As a result, a subtype of Mabry syndrome associated with mutations in the 1-6 mannosyltransferase 2, phosphatidylinositol glycan V (PIGV) gene was identified [Krawitz et al., 2010]. The inheritance of homozygous PIGV mutations disrupts the addition of the second mannose to the phosphatidylinositol glycan (GPI) anchor that is necessary to tether alkaline phosphatase at the plasma membrane.
Patients with PIGV mutations may have “non-cardinal” findings such as brachytelephalangy and anal anomalies [Marcelis et al., 2007; Horn et al., 2010; Horn et al., 2011] in addition to the trio of cardinal signs that includes elevated alkaline phosphatase. This contrasts with a group of patients with multiple congenital anomalies but no alkaline phosphatase involvement that are associated with mutations in the PIGN gene encoding a phosphoethanolamine transferase that acts at the first mannose of the GPI anchor [Maydan et al., 2011].
As a result, the heterogeneity of GPI deficiency disorders in general and of Mabry syndrome, in particular, is now becoming evident. Although variants of the (PIGV) gene have been identified in three families [Krawitz et al., 2010; Horn et al., 2011], mutations in PIGV have not been identified in other cases [Thompson et al., 2011]. In general, the PIGV gene disruptions appear to result in a broader phenotype [Horn et al., 2011] than as reported originally [Mabry et al., 1970]. In particular, patients with PIGV gene variants demonstrate brachytelephalangy, although the subject of our earlier case study [Thompson et al., 2006] is not among those cases associated with PIGV.
In this report, we present a series of four patients with hyperphosphatasia and neurologic deficit [Mabry et al., 1970]. While all four have the cardinal features of Mabry syndrome, only three have brachytelephalangy. Similar to previous studies [Krawitz et al., 2010; Horn et al., 2011], the cases with brachytelephalangy were found to harbor homozygous or compound heterozygous variants of the PIGV gene. The fourth case, lacking brachytelephalangy, was not associated with homozygous PIGV variation and may be considered similar to patients we presented previously [Thompson et al., 2010]. Mabry syndrome is discussed as a spectrum of phenotypic variability that reflects the diversity of GPI deficiency disorders.
CLINICAL REPORTS
Patient 1
This English girl of European ancestry is the younger sister of Patient 2. She was born to nonconsanguineous parents after a normal term pregnancy. Birth weight was 4.42 kg (99th centile) and head circumference was 38 cm (just over 99th centile). She was hypotonic at birth and had a round face, downturned mouth, and thickened helices.
The patient developed tonic-clonic seizures at age 8 weeks. Renal ultrasound study showed unilateral hydronephrosis. She had an anteriorly placed anus and constipation, but a rectal biopsy showed normal innervation. Development was delayed and at the age 4 years, she was still very hypotonic, not sitting and had no real language development. She had long palpebral fissures, a prominent nasal bridge, simple cupped ears with thickened helices, and a tented upper lip with downturned corners of the mouth (Fig. 1A).
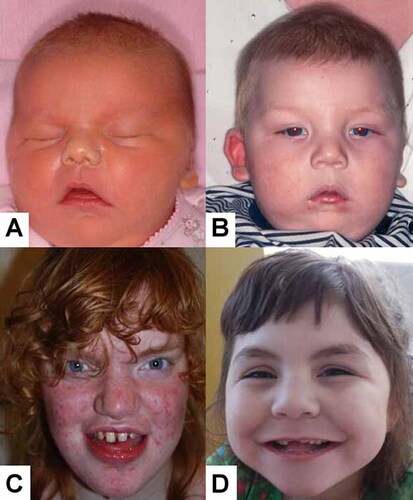
Composite showing the characteristics of: (A) An English girl, Patient 1, aged three days; (B) an English boy Patient 2, the brother of Patient 1, aged 7 years; (C) a Dutch girl, Patient 3, aged 17 years; (D) an American born girl, Patient 4, aged 6 years.
Brachytelephalangy was present with hypoplastic nails, especially on thumbs and little fingers. She developed a mild scoliosis. Alkaline phosphatase activity was increased to between 1,937 and 2,069 U/L (NR < 410 U/L). A karyotype was normal.
Patient 2
This English boy is the older sibling of Patient 1. The pregnancy was uneventful. An antenatal ultrasound scan at 35 weeks gestation showed bilateral renal pelvis dilatation. He was born by elective cesarean at term for breech presentation and had a birth weight of 4.98 kg and head circumference of 38 cm (both 99th centile). Seizures developed neonatally.
Soon after birth, he presented with abdominal distension and vomiting. A rectal biopsy showed short-segment Hirschsprung disease which required colostomy and anal dilatations. Bilateral supernumerary nipples were noted. Unilateral hydronephrosis was present.
He had simple cupped ears with thickened helices, a tented upper lip with downturned corners of the mouth, a high palate with bifid uvula, convergent squint, and glue ear but was considered to have satisfactory hearing.
Development was profoundly delayed at age 5 years. He had no speech, and could not sit unsupported although he smiled and occasionally fixed and followed. He was fully gastrostomy fed and had required a fundoplication for gastro-esophageal reflux. His height and weight followed the 50th to 75th centiles although his head circumference fell below the second centile.
Hypoplastic nails and diminutive terminal phalanges of all digits of both hands and feet suggested brachytelephalangy (Fig. 2A): which was confirmed on radiographs. He had problems swallowing secretions, and had multiple upper respiratory tract infections and was oxygen dependent. He died at 7 years of age.

Composite showing (A), Brachytelephalangy in the right hand of Patient 2 with missing or hypolastic nails; (B), radiograph of the right hand of Patient 3 showing brachytelephalangy.
Patient 3
This Dutch girl was the second child of consanguineous parents. During the pregnancy, oligohydramnios was noted and the ultrasound study showed kidney abnormalities. The patient was born at term and had a birth weight of 3,420 g (∼50th centile) with head circumference of 36 cm. She had a single umbilical artery.
At birth she was noted to have dysmorphic features including a tented upper lip. Hands and feet were affected with hypoplasia of the terminal phalanges and missing nails on the second and fifth digits. Moderate to severe intellectual disability, hypotonia and growth delay were noted at 2 years of age when the child was admitted to hospital for re-implantation of the ureters due to hydronephrosis and a hydroureter on the right. At that time she was also evaluated for developmental delay. She could stand and walk with support and spoke two words. She had no eye abnormalities and her hearing was normal. Seizures developed after the age of 5 years and these were treated with valproic acid. An MRI of the brain was normal. At the age of 10 years the alkaline phosphatase was noted to be very elevated (2,455 U/L).
Upon evaluation at the age of 17 years she was found to have a severe intellectual disability. Hyperventilation and hand-flapping occurred when she became excited. Weight, length, and skull circumference were normal. Her face showed a course appearance and she suffered from acne (Fig. 1C). She had hypertelorism with up-slanted palpebral fissures (inner canthal distance 3.6 cm, outer canthal distance 10.3 cm, and inter-pupillary distance 6.7 cm, all values ∼97th centile). The philtrum was short. There were multiple diastemata and prognathia was present. Radiographs of the hand confirmed brachytelephalangy (Fig. 2B). The alkaline phosphatase level was found to be 733 U/L. No distinctive abnormalities were noted on comparative genomic hybridization analysis (180 K Agilent array).
Patient 4
The Caucasian-American girl presented with developmental delay at age 16 months. She was the first child of healthy, nonconsanguineous parents, and had an unaffected younger sister. The pregnancy was uneventful and delivery was uncomplicated. Birth weight was 3,640 g (50th centile).
At the age of 1 year, she weighed 9 kg (25th centile), her head circumference was 47 cm (79th centile), and her height was 74.3 cm (50th centile).
Developmental delay was noted by the age of 16 months. Frequent generalized tonic-clonic seizures commenced at age 4 years. Treatment with valproic acid and rufinamide resulted in partial control of seizures. Cluster seizures were treated with clonazepam. Pyridoxine 100 mg was administered to treat possible pyridoxine-dependent seizures; however, there was no reduction in seizure frequency or severity.
On examination, her weight was 8.3 kg (less than the first centile), her length was 103.7 cm (25th centile), and her head circumference was 51.4 cm (50th centile). She had delayed psychomotor development and hypotonia, hypertelorism, a broad nasal bridge and tented upper lip. Her hands and feet were normal with no evidence of clinodactyly or brachytelephalangy.
Laboratory investigations for inborn disorders of metabolism, including urine and serum amino acids and lactate, were normal. The alkaline phosphatase level was elevated (2,000 U/L; NR < 400 U/L). A bone age was consistent with a slight delay of bone maturation. Her development continued to progress very slowly. Currently, she walks with support and understands simple commands but is unable to speak.
MOLECULAR GENETIC STUDIES
The PIGV gene coding region, intron-exon junctions and the putative promoter (NM_017837.2) were amplified from genomic DNA. PCR primers and conditions are available on request. Sequencing was performed using Applied Biosystems 3130/3170 Genetic Analyzers. Genbank sequences were downloaded from NCBI build 37.2 and mutation analysis was performed with Mutation Surveyor Software (SoftGenetics LLC, PA) or Sequencher 4.8 (GeneCodes Corp., MI).
The two siblings, Patients 1 and 2, were found to be compound heterozygotes for c.467G > A and c.494C > A in exon 3 of PIGV. The c.494C > A PIGV variant is reported here for the first time. Patient 3 was found to be compound heterozygous for the known c.1022C > A/c.1022C > T (p.Ala341Glu/p.Ala341Val) variants [Krawitz et al., 2010]. Patient 4 was found to be heterozygous for a c.1369C > T variation in exon 4 of the PIGV gene that results in a p.Leu457Phe substitution in the mannose transferase domain of the enzyme. Polyphen-2 modeling for pathogenicity of the p.Leu457Phe variant suggested that it would probably be damaging to protein structure and function (Polyphen score of 0.922; sensitivity of 0.44 and specificity of 0.97).
All of these mutations alter residues in PIGV that are well conserved from an evolutionary point of view. These mutations were not detected in the 4,000 exomes available through the University of Washington genome centre.
IMMUNOHISTOCHEMISTRY FOR CYTOPLASMIC INCLUSION BODIES
In Patients 1 and 2, no abnormal vacuoles were observed on rectal biopsy (data not shown). A 4 mm skin punch biopsy was performed in Patient 3. Fibroblasts cultured from the biopsy were grown in alpha-MEM media with 10% fetal bovine serum (FBS). Media was removed from confluent fibroblasts and the cells washed in PBS. Cultured cells were then examined using periodic acid Schiff (PAS) stain visualized using a 60× objective. Cytoplasmic inclusions were absent in fibroblasts taken from an unaffected control subject (Fig. 3A), sparsely distributed in fibroblasts taken from Patient 5 (Fig. 3B) of our previous study [Thompson et al., 2010] and clearly visible filling the cytoplasm in fibroblasts taken from Patient 3 (Fig. 3C). Routine electron microscopy examination of skin obtained from punch biopsy of patient 4 revealed no abnormalities (data not shown).
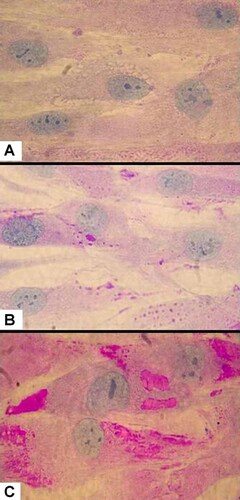
Composite showing the (100X) microscopy evidence for lysosomal storage in fibroblasts cultured from: (A) Patient 1; (B) non-PIGV patient reported previously [Thompson et al., 2010; Patient 5]; (C) unaffected patient. Periodic acid Schiff (PAS) stain and Haemotoxalin couter-stain was used to visualize the extent of glycolipid storage in the cytoplasm and the cell body and nucleus, respectively. Note that the cytoplasm of fibroblasts (A) are consolidated by vesicles stained with PAS; fewer vesicles stain with PAS are present in the non-PIGV case (B); fibroblasts from unaffected patients (C) show little PAS stain.
DISCUSSION
We report on four patients who present with findings typical of hyperphosphatasia with mental retardation (OMIM 239300), three of whom also present with brachytelephalangy. Inheritance and mutation segregation are consistent with a pattern of autosomal recessive transmission reported previously [Mabry et al., 1970]. All of the patients have experienced difficulty with seizure control; however, most are maintained on standard anticonvulsants. We note that, along with the French patient we presented previously [Thompson et al., 2010], seizures in Patient 4 did not respond to pyridoxine administration [Thompson et al., 2006; Stockler et al., 2011].
While the cases we presented previously highlighted the inter-case variability in alkaline phosphatase, the levels in these four patients were all elevated between three and five times the upper limit of normal, similar to those reported elsewhere [Horn et al., 2011]. In all cases, the characteristic findings are present as reported elsewhere [Horn et al., 2010; Thompson et al., 2010]. Facial dysmorphic features including hypertelorism with elongated palpebral fissures, broad nasal bridge, and tented upper lip with downturned corners of the mouth are common to all cases (Table I). Mutations in exon 3 of the PIGV gene (c.467G > A and c.494C > A) were associated with an extended phenotype in Patients 1 and 2. In addition to cup-shaped ears with thickened helices and a bifid uvula, one sibling had an anteriorly situated anus and the other had Hirschsprung disease not seen in all presentations of the syndrome [Marcelis et al., 2007; Krawitz et al., 2010; Thompson et al., 2010]. Brachytelephalangy [Gillessen-Kaesbach and Meinecke, 1999] is a distinctive feature in Patients 1 (Fig. 2A), 2 (data not shown), and 3 (Fig. 2B), but it is absent in Patient 4 (data not shown). Patients 1–3 had consistent hypoplasia of the phalanges in all fingers, shortened fingernails and thumbs.
| Specific findings | Mabrya | Patient 1b | Patient 2b | Patient 3 | Patient 4 |
|---|---|---|---|---|---|
| Clinical | |||||
| Normal pregnancy/birth history | +c | + | + | + | + |
| Consanguinity or affected siblings | + | + | + | + | − |
| Growth delay | (+)d | + | + | − | + |
| Head circumference | (N) | +e | +e | N | N |
| Facial abnormalities | (+) | + | + | + | + |
| Coarse features | (+) | − | − | + | – |
| Psychomotor retardation | SEVf | MOD | MOD | MOD/SEV | SEV |
| Hypotonia | ? | + | + | + | + |
| Seizures | + | + | + | + | + |
| Age of onset (years) | 2–3 | <1 | <1 | <2 | <2 |
| Pyridoxine responsiveness | ? | nd | nd | nd | – |
| Autistic behavior | ? | − | − | − | − |
| Multiple organ involvement/anal anomalies | ? | + | + | + | − |
| Biochemical | |||||
| Serum total ALP (n)g | 4.9–8.3 (?) | 5 (2) | 2.2 (1) | 4 (2) | 5 (2) |
| Tissue microscopy | |||||
| Intracellular inclusions | F; RB | − | − | F | − |
| PIGV: Compound heterozygous (1:2); heterozygous (0;1) | ? | 1:2 | 1:2 | 1:2 | 0:1 |
- N, normal; nd, not determined; RB, rectal biopsy; F, fibroblasts from skin biopsy. See text for further details.
- a Features described by Mabry et al. [1970].
- b Patients 1 and 2 are siblings. At birth, their head circumference was > + 2 SD; at 5 years of age < + 2 SD.
- c Feature present (+), feature absent (−), normal (N), unknown (?).
- d At least one of four patients was at the third centile at 13 years of age.
- e Father's head circumference is 60 cm (> + 2 SD).
- f SEV = severe; MOD = moderate [after Kruse et al., 1988].
- g ALP activity normalized by expressing value as a proportion of the age-specific upper limit of normal (×ULN), number of values given in parentheses (note: The number of determinations made by Mabry et al. [1970] in one case was not specified).
The phenotypic diversity among the four patients presented in this report and our previous series of five patients [Thompson et al., 2010] may be explained in part by the fact that fewer than half have homozygous or compound heterozygous mutations reported in the PIGV gene [Krawitz et al., 2010]. While Patient 4 from the original series [Thompson et al., 2010] and Patients 1, 2 and 3 from this series were found to be associated with homozygous inheritance of PIGV mutations (Table I), the remaining five patients were not found to have homozygous PIGV mutations. As with the extent of coarsening of facial features, the presence and extent of the bone abnormalities in the hands may be quite variable among the full spectrum of patients [Thompson et al., 2010] who present with the core syndrome [Mabry et al., 1970].
Further variability in the syndrome is underscored by the fact that the intracellular inclusions originally described [Mabry et al., 1970] may not be present in all cases [Thompson et al., 2010]. Our histological analysis identified inclusions in three previously reported cases [Thompson et al., 2010] and one case presented in this report. However, inclusions have yet to be identified in the remaining cases [Krawitz et al., 2010; Horn et al., 2011; Thompson et al., 2011].
In the present study, we identified inclusions in a child with Mabry syndrome with PIGV mutations. She was described to have coarse facial features often found in lysosomal storage disorders. We used PAS stain to identify the presence of absence of putative glycolipid stored in vacuoles. The appearance of cells from Patient 3 (Fig. 3C) is consistent with the presence of abundant cytoplasmic vacuoles (Fig. 3A). This differs from the patchy cytoplasmic vacuoles identified in fibroblasts cultured from a non-PIGV case [Thompson et al., 2010; Patient 5], and from scarce vacuoles in control fibroblasts (Fig. 3A).
As a result of this study, we suggest that Mabry syndrome should continue to be identified by the core trio of symptoms consisting of developmental disability, seizures, and hyperphosphatasia. Additional facial dysmorphic features as noted above may also suggest the diagnosis. Taken together with our previous study [Thompson et al., 2010], the cases presented indicate that in addition to the core signs, the phenotypic spectrum may include brachytelephalangy, anal abnormalities, and possibly other organ malformations [Horn et al., 2010; Horn et al., 2011].
Genetic heterogeneity is likely to explain this phenotypic variability—since PIGV mutations are found in only a subset of patients [Thompson et al., 2011]. The extended screening of infants with developmental delay, seizures, and dysmorphic features with or without brachytelephalangy (or other features) for elevated alkaline phosphatase, will assist in further delineating this condition. We suggest that future diagnostic approaches could make use of other phosphatidylinositol glycan-anchored enzymes—such as 5′-nucleotidase—in assessing the degree to which the GPI anchor is disrupted. The identification of mutations in the genes encoding other GPI anchor biosynthesis proteins may also extend the genotype–phenotype correlations for Mabry syndrome and related GPI deficiency disorders.
Acknowledgements
The authors would like to thank Dr. Chad Quarles and the members of all the families who continue to participate in the effort to help children with developmental disability. This study was supported by grants from the Scottish Rite Foundation of Canada and the Ontario Brain Institute (OBI) Integrated Discovery System: New Approaches to Intractable Epilepsy. An Epilepsy Canada Post Doctoral Fellowship was awarded to Miles Thompson. Support was provided to Frances Sharom by a Discovery grant from the Natural Sciences and Engineering Research Council of Canada. Support was provided to Tony Roscioli by the Australian NHMRC post-doctoral research fellowship.




