Fetal akinesia in metatropic dysplasia: The combined phenotype of chondrodysplasia and neuropathy?†
How to Cite this Article: Unger S, Lausch E, Stanzial F, Gillessen-Kaesbach G, Stefanova I, Di Stefano CM, Bertini E, Dionisi-Vici C, Nilius B, Zabel B, Superti-Furga A. 2011. Fetal akinesia in metatropic dysplasia: The combined phenotype of chondrodysplasia and neuropathy? Am J Med Genet Part A 155: 2860–2864.
Abstract
Dominant mutations in the receptor calcium channel gene TRPV4 have been associated with a family of skeletal dysplasias (metatropic dysplasia, pseudo-Morquio type 2, spondylometaphyseal dysplasia, Kozlowski type, brachyolmia, and familial digital arthropathy) as well as with dominantly inherited neuropathies (hereditary motor and sensory neuropathy 2C, scapuloperoneal spinal muscular atrophy, and congenital distal spinal muscular atrophy). While there is phenotypic overlap between the various members of each group, the two groups were considered to be totally separate with the former being strictly a structural skeletal condition and the latter group being confined to the peripheral nervous system. We report here on fetal akinesia as the presenting feature of severe metatropic dysplasia, suggesting that certain TRPV4 mutations can cause both a skeletal and a neuropathic phenotype. Three cases were detected on prenatal ultrasound because of absent movements in the second trimester. Case 4 presented with multiple joint contractures and absent limb movements at birth and was diagnosed with “fetal akinesia syndrome”. Post-interruption and post-natal X-rays showed typical features of metatropic dysplasia in all four. Sequencing of the TRPV4 gene confirmed the presence of de novo heterozygous mutations predicting G78W (Case 1), T740I (Cases 2 and 3), and K276E (Case 4). Although some degree of restriction of movements is not uncommon in fetuses with skeletal dysplasia, akinesia as leading sign is unusual and suggests that certain TRPV4 mutations produce both chondrodysplasia and a peripheral neuropathy resulting in a severe “overlap” phenotype. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
In 2008, it was discovered that mutations in the gene encoding the transient receptor potential cation channel, subfamily V, member 4 (TRPV4) were responsible for the relatively mild and rare skeletal dysplasia, brachyolmia (OMIM 113500) [Rock et al., 2008]. As the name implies, the skeletal abnormalities in brachyolmia are essentially limited to the spine. However, through comparison of radiographs and clinical findings, TRPV4 was considered a likely candidate gene for two more generalized skeletal dysplasias: Spondylometaphyseal dysplasia (SMD)-Kozlowski type (OMIM 184252) and metatropic dysplasia (OMIM 156530). Indeed, TRPV4 mutations have now been found in multiple patients with SMD-Kozlowski and metatropic dysplasia [Krakow et al., 2009] as well as parastremmatic dysplasia (OMIM 168400) and spondyloepimetaphyseal dysplasia (SEMD)-Maroteaux type (also known as Pseudo-Morquio type 2) [Nishimura et al., 2010] establishing the “TRPV4-family” of skeletal dysplasias ranging from mild to lethal [Warman et al., 2011].
Unexpectedly, heterozygous TRPV4 mutations have also been found to account for congenital distal spinal muscular atrophy (SMA) (OMIM 600175), scapuloperoneal SMA (OMIM 181405), and hereditary motor and sensory neuropathy type 2C (HMSN2C) (MIM 606071) [Auer-Grumbach et al., 2010; Deng et al., 2010; Landouré et al., 2010]. These conditions can also be viewed as members of a “TRPV4-family” as although overlapping, the phenotypes are not exactly the same. Interestingly, different diagnostic labels have been applied to various individuals within a family segregating a neuropathy phenotype [Auer-Grumbach et al., 2010].
For the most part, the TRPV4 mutations reported in the neurological phenotype patients are different than those reported in the patients with skeletal disorders but there is no clear explanation as to why the mutations should have such distinct effects [Dai et al., 2010a]. However, it has been proposed that the two groups of disorders are distinct [Dai et al., 2010a]. We report four patients with a clear radiographic diagnosis of metatropic dysplasia but whose presenting feature was akinesia/arthrogryposis demonstrating that the dual neurological–skeletal phenotype sometimes occur simultaneously.
CLINICAL REPORTS
Case 1: The parents of this fetus were both healthy and nonconsanguineous. This was the mother's third pregnancy and she had one healthy daughter and son. On routine ultrasound in the 20th gestational week multiple abnormalities were noted. These included short long bones, predominantly affecting the femurs, narrow bell-shaped thorax, finger contractures, and undetectable fetal movements. An amniocentesis was preformed and the karyotype was normal (46, XY). On the basis of the ultrasound findings, the pregnancy was interrupted in the 21st week. On subsequent clinical examination, the fetus was noted to have short upper and lower extremities, cartilaginous expansions of the elbow, wrist, and knee joints, finger contractures, and relatively long hands and feet (Fig. 1A). Radiographic examination revealed short long bones with mildly accentuated metaphyses, except at the humeri where the metaphyseal expansion was more marked. There was a narrow thorax with an abnormal contour and those vertebral bodies that could be assessed were markedly flat (Fig. 1B).
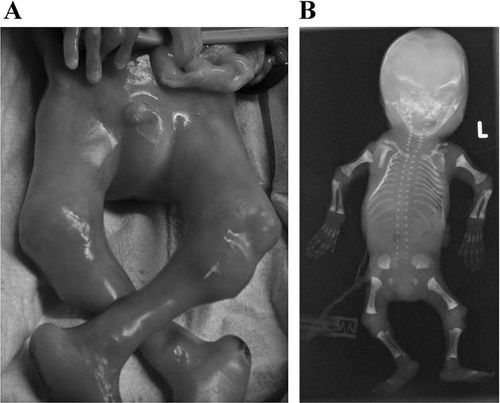
A: This photograph of the lower limbs of Patient 1 shows the marked expansion of the joints. The most obvious enlargement is of the proximal tibia. There are also rocker bottom feet. B: This AP fetogram of Patient 1 also shows metaphyseal expansion but it is less dramatic than that observed on clinical photos indicating that much of the expansion is cartilaginous in origin. An assessment of the right iliac wing is difficult due to rotation but on the left there is an abnormal shape with a narrow sacrosciatic notch and the beginning of a halberd appearance. Many of the vertebral bodies are not ossified but in the mid-thoracic region severe platyspondyly is appreciable.
Cases 2 and 3 (fetus 1 and 2): This twin pregnancy was the third for this healthy nonconsanguineous couple. They have one older son and the mother had one spontaneous abortion at 11 weeks. The twins were diamniotic dichorionic but post-mortem analysis of polymorphisms revealed that it was a monozygotic pregnancy. Absence of fetal movements with arthrogryposis was detected on 20-week ultrasound. At the same time, both fetuses were noted to have short long bones and fetus 2 had an atrioventricular canal and generalized hydrops. Based on the poor prognosis, the parents opted to terminate the pregnancy. Autopsy was performed on both fetuses. In fetus 1, thoracic hypoplasia and short limbs with contractures were confirmed on post-mortem examination and the presence of a sacrococcygeal “tail” was noted. Fetus 1 was also found to have a ventricular septal defect with a diameter of 2 mm and aortic isthmus stenosis. Fetus 2 had nearly identical external physical features, including the presence of the tail (Fig. 2A and B). The presence of an atrioventricular canal was confirmed and an aortic isthmus stenosis was found. Radiographs were made of both fetuses (Fig. 2C and D) and demonstrated findings diagnostic of metatropic dysplasia.
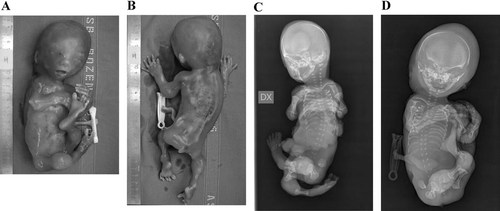
A: The position of this fetus is unchanged from in utero and is indicative of the contractures/akinesia. There is enlargement of the large joints most readily visible in the left elbow and knee. B: In this view, the enlargement of the wrists and ankles are also clearly visible. There is a sacrococcygeal tail. C: The position of the limbs is indicative of the absence of fetal movements. The osseous features are typical of severe metatropic dysplasia. The spine is apparently elongated but there is platyspondyly. The diaphyses of the long bones are fairly normal but the metaphyses are ballooned. D: The radiographic changes are similar to those seen in the sib. The position of the lower limbs is also indicative of akinesia. The thorax is long and narrow. It is probable that the shape of the thorax would be incompatible with life.
Case 4: The parents of this boy are a consanguineous couple of Algerian origin. They had one healthy daughter. During the pregnancy, the mother noted diminished fetal movements and this was confirmed by ultrasound that also showed fixed internally rotated feet. The child was born by elective cesarean at 36-weeks gestation and required a short course of intubation but was successfully extubated following a single administration of surfactant. His birthweight was 2.3 kg and head circumference was 35 cm but length was not possible to determine accurately due to contractures. On physical examination, there was thoracic hypoplasia, clubbed feet, camptodactly, and the appearance of enlarged joints. He also had bilateral inguinal hernias and a small umbilical hernia. The legs could not be straightened and remained in a flexed and adducted position. The neonatal neurological examination, as much as was possible given the restriction of movements was normal. An EMG was performed at 3 months of age and showed an absence of voluntary activity in the lower limbs. There was some residual activity in the upper limbs (deltoid examined) but with signs of a chronic axonal denervating process considered to be indicative of a neuropathic disorder. The baby died of respiratory complications at age 4 months. Neonatal radiographs showed the abnormalities typical of severe metatropic dysplasia (Fig. 3).
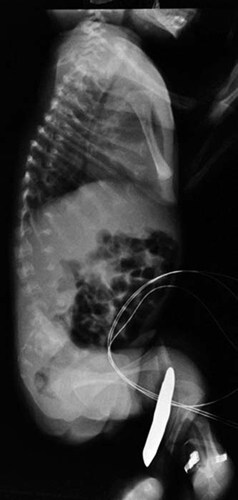
A lateral radiograph of the thorax and spine of Patient 4 shows the marked platyspondyly but widened intervertebral spaces. The ribs are short and this diminished thoracic capacity undoubtedly contributed to the patient's demise secondary to respiratory infection.
METHODS
Genomic DNA was extracted by standard procedures from cultured amniocytes (Case 1), fibroblasts (Cases 2 and 3), and peripheral blood (Case 4) using standard protocols. The exons and flanking intronic sequences of TRPV4 were sequenced as previously described [Nishimura et al., 2010]. All mutations were confirmed by a second PCR and sequencing and parental DNA was available for all.
RESULTS
All four individuals were found to have heterozygous mutations in TRPV4. Unsurprisingly, the monozygotic twins (Cases 2 and 3) carried the same mutation. The three mutations predicted missense changes (G78W, T740I, and K276E) and none were found in the clinically unaffected parents. These particular mutations have not been previously reported in either skeletal or neurological disorders associated with TRPV4 [Andreucci et al., 2011; Dai et al., 2010b].
DISCUSSION
We report on four patients with TRPV4 mutations and the dual phenotype of metatropic dysplasia and fetal akinesia. The mutations (G78W, K276E, and T740I) were not previously described but are likely to be pathogenic based on amino acid conservation between species and their de novo appearance.
Since the discovery of heterozygous TRPV4 mutations in brachyolmia in 2008 [Rock et al., 2008], a variety of mutations have been reported in what is referred to now as the “TRPV4 group” [Andreucci et al., 2011; Dai et al., 2010b; Krakow et al., 2009]. The skeletal dysplasia family includes brachyolmia, metatropic dysplasia, SEMD, Maroteaux type, SMD, Kozlowski type, and familial digital arthropathy with brachydactyly [Warman et al., 2011]. In 2010, mutations in TRPV4 were recognized as causative for a family of neurological disorders. The mutations were distinct from those observed in patients with skeletal phenotypes although the pathogenesis of this division was not clear and remains subject to debate and functional studies [Auer-Grumbach et al., 2010; Deng et al., 2010; Landouré et al., 2010; Loukin et al., 2011]. Most mutations have been missense mutations with the exception of F471del reported in a patient with metatropic dysplasia [Dai et al., 2010b] and a frameshift mutation in a patient with SEMD, Maroteaux type [Nishimura et al., 2010]. It is not obvious why some mutations result in one phenotype over another but the intriguing possibility that certain mutations associated with the neurological phenotype may predispose the TRPV4 transcript to act like another TRPV isoform (trans-speciation) [Dai et al., 2010a]. Recent evidence suggests that the mutations associated with a skeletal phenotype cause increased basal activity of the calcium channel [Loukin et al., 2011]. However, the same has been shown for the mutations associated with the clear neurological phenotype [Fecto et al., 2011].
In a manner, it was reminiscent of the situation with FLNA. Mutations in this gene could produce either a skeletal phenotype (frontometaphyseal dysplasia, osteodysplasty Melnick-Needles, oto-palato-digital syndrome types 1 and 2) or a neurological one (periventricular nodular heterotopias). The mutations were considered to have different mechanisms of action. However, in 2004, a patient was described with both the neurological and skeletal phenotypes caused by a particular FLNA mutation [Zenker et al., 2004]. It was proposed that the mutation led to two different abnormal transcripts with divergent functions.
A patient with SMD, Kozlowski type, and a CMT has recently been described [Cho et al., 2011]. This particular mutation had previously been seen in patients with presumably isolated SMD, Kozlowski type, although it is possible that neuromuscular symptoms might have been under investigation with the presumption that they were simply secondary to the skeletal dysplasia. Another patient has been reported with mild CMT2C and platyspondyly with short stature. That family had a previously unreported mutation (S542Y) [Chen et al., 2010]. As our patients have so far unreported mutations, it is conceivable that specific TRPV4 mutations may indeed result in a combined, or hybrid, phenotype; this hypothesis will have to be confirmed or refuted with more observations.
Our series of patients had a distinctive neurological phenotype of large joint contractures presenting as fetal akinesia. Absence of fetal movements can have a variety of causes from central nervous system abnormalities through to muscular or joint abnormalities [Bayat et al., 2009]. However, skeletal dysplasias generally do not cause arthrogryposis multiplex. In our one patient (Case 4) in whom electrophysiological studies were possible, there was clear evidence of a neuropathic process on EMG. This supports the hypothesis that, at least in some patients, a TRPV4 mutation can cause a severe skeletal dysplasia and a neurological phenotype characterized by congenital contractures. Of note, congenital contractures are often seen in distal SMA but the muscle weakness has been regarded as the prominent feature [Auer-Grumbach et al., 2010]. This current series of patients expands our conception of the spectrum of neurological consequences of TRPV4 mutation but it remains to be seen if the phenotype of fetal akinesia is always linked with metatropic dysplasia or can occur in isolation. However, with the rapid evolution of high throughput sequencing technologies and their increasing clinical use, it would seem prudent to include TRPV4 in the panel of genes to be sequenced for fetal akinesia/congenital contractures/arthrogryposis.




